Interventions for Old World cutaneous leishmaniasis
- PMID: 29149474
- PMCID: PMC6486265
- DOI: 10.1002/14651858.CD005067.pub4
Interventions for Old World cutaneous leishmaniasis
Update in
-
Interventions for Old World cutaneous leishmaniasis.Cochrane Database Syst Rev. 2017 Dec 1;12(12):CD005067. doi: 10.1002/14651858.CD005067.pub5. Cochrane Database Syst Rev. 2017. PMID: 29192424 Free PMC article.
Abstract
Background: Cutaneous leishmaniasis, caused by a parasitic infection, is considered one of the most serious skin diseases in many low- and middle-income countries. Old World cutaneous leishmaniasis (OWCL) is caused by species found in Africa, Asia, the Middle East, the Mediterranean, and India. The most commonly prescribed treatments are antimonials, but other drugs have been used with varying success. As OWCL tends to heal spontaneously, it is necessary to justify the use of systemic and topical treatments. This is an update of a Cochrane Review first published in 2008.
Objectives: To assess the effects of therapeutic interventions for the localised form of Old World cutaneous leishmaniasis.
Search methods: We updated our searches of the following databases to November 2016: the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase, and LILACS. We also searched five trials registers and checked the reference lists of included studies for further references to relevant randomised controlled trials (RCTs). We wrote to national programme managers, general co-ordinators, directors, clinicians, WHO-EMRO regional officers of endemic countries, pharmaceutical companies, tropical medicine centres, and authors of relevant papers for further information about relevant unpublished and ongoing trials. We undertook a separate search for adverse effects of interventions for Old World cutaneous leishmaniasis in September 2015 using MEDLINE.
Selection criteria: Randomised controlled trials of either single or combination treatments in immunocompetent people with OWCL confirmed by smear, histology, culture, or polymerase chain reaction. The comparators were either no treatment, placebo/vehicle, and/or another active compound.
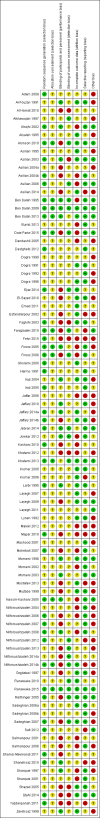
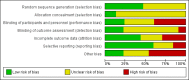
Data collection and analysis: Two review authors independently assessed trials for inclusion and risk of bias and extracted data. We only synthesised data when we were able to identify at least two studies investigating similar treatments and reporting data amenable to pooling. We also recorded data about adverse effects from the corresponding search.
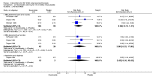
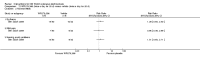
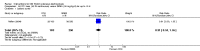
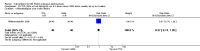
Main results: We included 89 studies (of which 40 were new to this update) in 10,583 people with OWCL. The studies included were conducted mainly in the Far or Middle East at regional hospitals, local healthcare clinics, and skin disease research centres. Women accounted for 41.5% of the participants (range: 23% to 80%). The overall mean age of participants was 25 years (range 12 to 56). Most studies lasted between two to six months, with the longest lasting two years; average duration was four months. Most studies were at unclear or high risk for most bias domains. A lack of blinding and reporting bias were present in almost 40% of studies. Two trials were at low risk of bias for all domains. Trials reported the causative species poorly.Here we provide results for the two main comparisons identified: itraconazole (200 mg for six to eight weeks) versus placebo; and paromomycin ointment (15% plus 10% urea, twice daily for 14 days) versus vehicle.In the comparison of oral itraconazole versus placebo, at 2.5 months' follow up, 85/125 participants in the itraconazole group achieved complete cure compared to 54/119 in the placebo group (RR 3.70, 95% CI 0.35 to 38.99; 3 studies; 244 participants). In one study, microbiological or histopathological cure of skin lesions only occurred in the itraconazole group after a mean follow-up of 2.5 months (RR 17.00, 95% CI 0.47 to 612.21; 20 participants). However, although the analyses favour oral itraconazole for these outcomes, we cannot be confident in the results due to the very low certainty evidence. More side effects of mild abdominal pain and nausea (RR 2.36, 95% CI 0.74 to 7.47; 3 studies; 204 participants) and mild abnormal liver function (RR 3.08, 95% CI 0.53 to 17.98; 3 studies; 84 participants) occurred in the itraconazole group (as well as reports of headaches and dizziness), compared with the placebo group, but again we rated the certainty of evidence as very low so are unsure of the results.When comparing paromomycin with vehicle, there was no difference in the number of participants who achieved complete cure (RR of 1.00, 95% CI 0.86, 1.17; 383 participants, 2 studies) and microbiological or histopathological cure of skin lesions after a mean follow-up of 2.5 months (RR 1.03, CI 0.88 to 1.20; 383 participants, 2 studies), but the paromomycin group had more skin/local reactions (such as inflammation, vesiculation, pain, redness, or itch) (RR 1.42, 95% CI 0.67 to 3.01; 4 studies; 713 participants). For all of these outcomes, the certainty of evidence was very low, meaning we are unsure about these results.Trial authors did not report the percentage of lesions cured after the end of treatment or speed of healing for either of these key comparisons.
Authors' conclusions: There was very low-certainty evidence to support the effectiveness of itraconazole and paromomycin ointment for OWCL in terms of cure (i.e. microbiological or histopathological cure and percentage of participants completely cured). Both of these interventions incited more adverse effects, which were mild in nature, than their comparisons, but we could draw no conclusions regarding safety due to the very low certainty of the evidence for this outcome.We downgraded the key outcomes in these two comparisons due to high risk of bias, inconsistency between the results, and imprecision. There is a need for large, well-designed international studies that evaluate long-term effects of current therapies and enable a reliable conclusion about treatments. Future trials should specify the species of leishmaniasis; trials on types caused by Leishmania infantum, L aethiopica, andL donovani are lacking. Research into the effects of treating women of childbearing age, children, people with comorbid conditions, and those who are immunocompromised would also be helpful.It was difficult to evaluate the overall efficacy of any of the numerous treatments due to the variable treatment regimens examined and because RCTs evaluated different Leishmania species and took place in different geographical areas. Some outcomes we looked for but did not find were degree of functional and aesthetic impairment, change in ability to detect Leishmania, quality of life, and emergence of resistance. There were only limited data on prevention of scarring.
Conflict of interest statement
Julio Heras‐Mosteiro: none known. Begoña Monge‐Maillo: none known. Mariona Pinart: none known. Patricia Lopez Pereira: none known. Emely Garcia‐Carrasco: none known. Pedro Campuzano Cuadrado: none known. Ana Royuela: none known. Irene Mendez Roman: none known. Rogelio López‐Vélez: none known.
Iraj Sharifi, one of the clinical referees, was a co‐author on the included studies Khatami 2012 and Daie Parizi 2015.
Figures











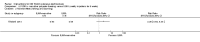
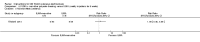










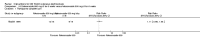






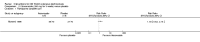






























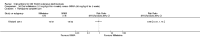






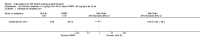








































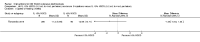














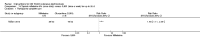

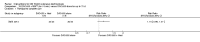
















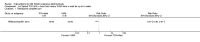


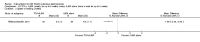














































Update of
-
Interventions for Old World cutaneous leishmaniasis.Cochrane Database Syst Rev. 2008 Oct 8;(4):CD005067. doi: 10.1002/14651858.CD005067.pub3. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2017 Nov 17;11:CD005067. doi: 10.1002/14651858.CD005067.pub4. PMID: 18843677 Updated.
References
References to studies included in this review
-
- Adam I, Hagelnur A. Artesunate plus sulfamethoxypyrazine/pyrimethamine for the treatment of cutaneous leishmaniasis: a double‐blind, placebo‐controlled clinical trial. International Journal of Antimicrobial Agents 2009;34(4):380‐1. [PUBMED: 19409761 ] - PubMed
-
- al‐Fouzan AS, al‐Saleh QA, Najem NM, Rostom AI. Cutaneous leishmaniasis in Kuwait. Clinical experience with itraconazole. International Journal of Dermatology 1991;30(7):519‐21. [PUBMED: 1663089] - PubMed
-
- Al Hamdi K, Awad AH, Moker HM. Evaluation of intralesional 0.2% ciprofloxacin as a treatment for cutaneous leishmaniasis. Eastern Mediterranean Health Journal 2010;16(1):89‐93. [PUBMED: 20214164] - PubMed
-
- Alkhawajah AM, Larbi E, al‐Gindan Y, Abahussein A, Jain S. Treatment of cutaneous leishmaniasis with antimony: intramuscular versus intralesional administration. Annals of Tropical Medicine and Parasitology 1997;91(8):899‐905. [PUBMED: 9579209] - PubMed
-
- Alrajhi AA, Ibrahim EA, Vol EB, Khairat M, Faris RM, Maguire JH. Fluconazole for the treatment of cutaneous leishmaniasis caused by leishmania major. New England Journal of Medicine 2002;346(12):891‐5. [PUBMED: 11907288] - PubMed
References to studies excluded from this review
-
- Alavi‐Naini R, Fazaeli A, O'Dempsey T. Topical treatment modalities for old world cutaneous leishmaniasis: a review. Prague Medical Report 2012;113(2):105‐18. [PUBMED: 22691282] - PubMed
-
- Banihashemi M, Yazdanpanah MJ, Amirsolymani H, Yousefzadeh H. Comparison of lesion improvement in lupoid leishmaniasis patients with two treatment approaches: trichloroacetic Acid and intralesional meglumine antimoniate. Journal of Cutaneous Medicine and Surgery 2015;19(1):35‐9. [PUBMED: 25775661] - PubMed
-
- Bumb RA, Mehta RD, Ghiya BC, Jakhar R, Prasad N, Soni P, et al. Efficacy of short‐duration (twice weekly) intralesional sodium stibogluconate in treatment of cutaneous Leishmaniasis in India. British Journal of Dermatology 2010;163(4):854‐8. [PUBMED: 20500797] - PubMed
-
- Dogra J, Lal BB, Misra SN. Dapsone in the treatment of cutaneous leishmaniasis. International Journal of Dermatology 1986;25(6):398‐400. [PUBMED: 3531044] - PubMed
-
- Dogra J, Aneja N. Leishmaniasis and itraconazole: a controlled clinical trial on cutaneous subtypes. International Journal of Antimicrobial Agents 1994;4(4):309‐11. [PUBMED: 18611622] - PubMed
References to studies awaiting assessment
-
- Farajzadeh S, Hakimi Parizi M, Haghdoost AA, Mohebbi A, Mohammadi S, Pardakhty A, et al. Comparison between intralesional injection of zinc sulfate 2 % solution and intralesional meglumine antimoniate in the treatment of acute old world dry type cutaneous leishmaniasis: a randomized double‐blind clinical trial. Journal of Parasitic Diseases 2016;40(3):935‐9. [PUBMED: 27605813] - PMC - PubMed
-
- Farajzadeh S, Heshmatkhah A, Vares B, Mohebbi E, Mohebbi A, Aflatoonian M, et al. Topical terbinafine in the treatment of cutaneous leishmaniasis: triple blind randomized clinical trial. Journal of Parasitic Diseases 2016;40(4):1159‐64. [DOI: 10.1007/s12639-014-0641-1; PUBMED: 27876906] - DOI - PMC - PubMed
-
- Hanif MM, Akram K, Mustafa G. Intralesional versus oral chloroquine in cutaneous leishmaniasis: comparison of outcome, duration of treatment and total dose of drug. Journal of the College of Physicians and Surgeons Pakistan 2016;26(4):260‐2. [PUBMED: 27097693] - PubMed
-
- Jaffary F, Nilforoushzadeh MA, Siadat A, Haftbaradaran E, Ansari N, Ahmadi E. A comparison between the effects of Glucantime, topical trichloroacetic acid 50% plus Glucantime, and fractional carbon dioxide laser plus Glucantime on cutaneous leishmaniasis lesions. Dermatology Research and Practice 2016;2016:6462804. [PUBMED: 27148363] - PMC - PubMed
-
- Na‐Bangchang K, Ahmed O, Hussein J, Hirayama K, Kongjam P, Aseffa A, et al. Exploratory, phase II controlled trial of shiunko ointment local application twice a day for 4 weeks in Ethiopian patients with localized cutaneous leishmaniasis. Evidence‐Based Complementary and Alternative Medicine 2016;2016:5984709. [PUBMED: 27195014] - PMC - PubMed
References to ongoing studies
-
- ACTRN12614001288617. A clinical trial to assess the safety and effect of heat therapy in comparison to standard intra‐lesional sodium stibogluconate for cutaneous leishmaniasis [In patients with cutaneous leishmaniasis, thermotherapy was compared with standard intra‐lesional therapy with regard to efficacy and safety]. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12614001288617 Date first received: 10 December 2014.
-
- IRCT138904091159N7. Comparison between two groups in the treatment of cutaneous leishmaniasis [Comparison between the efficacy of intralesional placebo and nitric oxide releasing patch versus placebo patch and glucantime in the treatment of cutaneous leishmaniasis]. www.irct.ir/searchresult.php?id=1159&number=7 Date first received: 2 September 2013.
-
- IRCT2013092414746N1. Treatment of patients with ZCL [The effect of MJ1 (Topical dairy extract) versus routine care for treatment of cutaneous leishmaniasis (rural) in Isfahan Iran :a randomized controled clinical trial (RCTs) ‐]. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2013092414746N1 Date first received: 20 February 2014.
-
- NCT00840359. Study of the Efficacy of Daylight Activated Photodynamic Therapy in the Treatment of Cutaneous Leishmaniasis [Phase 2 Study of the Efficacy of Daylight Activated Photodynamic Therapy in the Treatment of Cutaneous Leishmaniasis]. clinicaltrials.gov/ct2/show/NCT00840359 Date first received: 8 February 2009.
-
- NCT01050777. Efficacy of Topical Liposomal Form of Drugs in Cutaneous Leishmaniasis [Pilot Study of Efficacy of Topical Nano‐liposomal Meglumine Antimoniate (Glucantime) or Paromomycin in Combination With Systemic Glucantime for the Treatment of Anthroponotic Cutaneous Leishmaniasis (ACL) Caused by Leishmania Tropica]. clinicaltrials.gov/ct2/show/NCT01050777 Date first received: 13 January 2010.
Additional references
-
- Adam I, Elhardello OA, Elhadi MO, Abdalla E, Elmardi KA, Jansen FH. The antischistosomal efficacies of artesunate–sulfamethoxypyrazine–pyrimethamine and artemether–lumefantrine administered as treatment for uncomplicated Plasmodium falciparum malaria. Annals of Tropical Medicine and Parasitology 2008;102(1):39‐44. [PUBMED: 18186976] - PubMed
-
- Al‐Waiz M, Sharquie KE, Al‐Assir M. Treatment of cutaneous leishmaniasis by intralesional metronidazole. Saudi Medical Journal 2004;25(10):1512‐3. [PUBMED: 15494841] - PubMed
-
- Alrajhi AA. Cutaneous leishmaniasis of the Old World. Skin Therapy Letter 2003;8(2):1‐4. [PUBMED: 12728282] - PubMed
-
- Alvar J, Yactayo S, Bern C. Leishmaniasis and poverty. Trends in Parasitology 2006;22(12):552‐7. [PUBMED: 17023215] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

