Sterol Oxidation Mediates Stress-Responsive Vms1 Translocation to Mitochondria
- PMID: 29149595
- PMCID: PMC5837041
- DOI: 10.1016/j.molcel.2017.10.022
Sterol Oxidation Mediates Stress-Responsive Vms1 Translocation to Mitochondria
Abstract
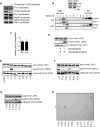
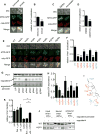
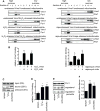
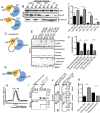
Vms1 translocates to damaged mitochondria in response to stress, whereupon its binding partner, Cdc48, contributes to mitochondrial protein homeostasis. Mitochondrial targeting of Vms1 is mediated by its conserved mitochondrial targeting domain (MTD), which, in unstressed conditions, is inhibited by intramolecular binding to the Vms1 leucine-rich sequence (LRS). Here, we report a 2.7 Å crystal structure of Vms1 that reveals that the LRS lies in a hydrophobic groove in the autoinhibited MTD. We also demonstrate that the oxidized sterol, ergosterol peroxide, is necessary and sufficient for Vms1 localization to mitochondria, through binding the MTD in an interaction that is competitive with binding to the LRS. These data support a model in which stressed mitochondria generate an oxidized sterol receptor that recruits Vms1 to support mitochondrial protein homeostasis.
Keywords: ROS signaling; S. cerevisiae; biochemistry; lipid signaling; liposomes; mitochondrial quality control; oxidative stress; protein degradation; sterols; structure-function.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

