Prostaglandin J2 promotes O-GlcNAcylation raising APP processing by α- and β-secretases: relevance to Alzheimer's disease
- PMID: 29149631
- PMCID: PMC5743611
- DOI: 10.1016/j.neurobiolaging.2017.10.009
Prostaglandin J2 promotes O-GlcNAcylation raising APP processing by α- and β-secretases: relevance to Alzheimer's disease
Abstract
Regulation of the amyloid precursor protein (APP) processing by α- and β-secretases is of special interest to Alzheimer's disease (AD), as these proteases prevent or mediate amyloid beta formation, respectively. Neuroinflammation is also implicated in AD. Our data demonstrate that the endogenous mediator of inflammation prostaglandin J2 (PGJ2) promotes full-length APP (FL-APP) processing by α- and β-secretases. The decrease in FL-APP was independent of proteasomal, lysosomal, calpain, caspase, and γ-secretase activities. Moreover, PGJ2-treatment promoted cleavage of secreted APP, specifically sAPPα and sAPPβ, generated by α and β-secretase, respectively. Notably, PGJ2-treatment induced caspase-dependent cleavage of sAPPβ. Mechanistically, PGJ2-treatment selectively diminished mature (O- and N-glycosylated) but not immature (N-glycosylated only) FL-APP. PGJ2-treatment also increased the overall levels of protein O-GlcNAcylation, which occurs within the nucleocytoplasmic compartment. It is known that APP undergoes O-GlcNAcylation and that the latter protects proteins from proteasomal degradation. Our results suggest that by increasing protein O-GlcNAcylation levels, PGJ2 renders mature APP less prone to proteasomal degradation, thus shunting APP toward processing by α- and β-secretases.
Keywords: APP; Apoptosis; O-GlcNAcylation; Prostaglandin J2; Secretases.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures





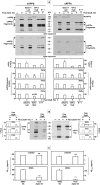

 ). PGJ2-treatment did not alter the intracellular levels of Aβ, while it decreased the levels of secreted Aβ. The scheme depicts the complexity of APP metabolism, rendering it difficult to analyze stoichiometrically. For simplicity, the CTF fragments are not shown under PGJ2-treatment. O-GlcNAc, O-linked β-N-acetylglucosamine.
). PGJ2-treatment did not alter the intracellular levels of Aβ, while it decreased the levels of secreted Aβ. The scheme depicts the complexity of APP metabolism, rendering it difficult to analyze stoichiometrically. For simplicity, the CTF fragments are not shown under PGJ2-treatment. O-GlcNAc, O-linked β-N-acetylglucosamine.References
-
- Aoki T, Narumiya S. Prostaglandins and chronic inflammation. Trends Pharmacol Sci. 2012;33:304–311. - PubMed
-
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, Xie X, Blazey TM, Holtzman DM, Santacruz A, Buckles V, Oliver A, Moulder K, Aisen PS, Ghetti B, Klunk WE, McDade E, Martins RN, Masters CL, Mayeux R, Ringman JM, Rossor MN, Schofield PR, Sperling RA, Salloway S, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. - PMC - PubMed
-
- Benzing WC, Mufson EJ, Armstrong DM. Alzheimer’s disease-like dystrophic neurites characteristically associated with senile plaques are not found within other neurodegenerative diseases unless amyloid beta-protein deposition is present. Brain Res. 1993;606:10–18. - PubMed
-
- Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 1993;303:474–482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

