Biobanking in Israel 2016-17; expressed perceptions versus real life enrollment
- PMID: 29149849
- PMCID: PMC5693555
- DOI: 10.1186/s12910-017-0223-8
Biobanking in Israel 2016-17; expressed perceptions versus real life enrollment
Abstract
Background: As part of the preparations to establish a population-based biobank in a large Israeli health organization, we aimed to investigate through focus groups the knowledge, perceptions and attitudes of insured Israelis, toward biobanking, and then, after input from focus groups' participants, to empirically assess the impact of a revised recruitment process on recruitment rates.
Methods: 1) Six Focus group discussions were conducted (n = 10 per group) with individuals who had routine blood laboratory tests taken in the last 2 years. 2) After addressing the issues raised in the focus groups and revising the recruitment process, individuals undergoing routine blood tests in phlebotomy clinics (N = 10,262) were invited to participate in the future biobank.
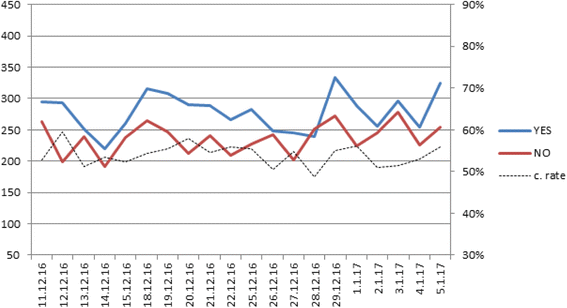
Results: At the outset of the focus groups there was an overall positive response to the prospect of a population-based biobank. Concerns revolved around infringement on privacy, fears of the "big brother"(e.g. insurance companies), and anxiety about inequality. Reaction to the language of the informed consent document revolved around concerns over ability to maintain anonymity, to withdraw consent, involvement of commercial entities, and the general tenor of the informed consent, which was perceived as legalistic and unilateral. In general, the longer participants were exposed to discussion about the biobank, the less likely they were to consent to sign in. Overall, only 20% (12) of the 60 participants stated they would agree to sign in by the end of the 2 hour group session. The feedback obtained from the focus groups was used in the second stage ("real life") of the study. A team of recruiters received extensive training to enable fruitful discussion and a detailed explanation to questions and concerns raised during the recruitment process. During the second stage of the study, after revising the consent form and training recruiters, a 53% consent rate was observed among 10,262 participants, more than 4 fold higher than estimated at the focus group stage.
Conclusions: The qualitative focus group research helped identify important perceptions and concerns, which were subsequently addressed in the revised consent form and in the discussion the recruiters had with potential biobank donors.
Keywords: Biobanking; Informed consent form; Population based biobanking; Risk perception; Volunteers.
Conflict of interest statement
Ethics approval and consent to participate
Approval was received from Assuta Hospital, Tel Aviv, Israel. All participants gave written informed consent to participate.
Consent for publication
Not applicable.
Competing interests
The study was supported financially by Teva Inc. and Pfizer Inc. The funding bodies did not participate in the design of the study or interpretation of the results. Teva Inc. reviewed the manuscript and suggested revisions to language and references, but not to interpretation of results.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Participatory improvement of a template for informed consent documents in biobank research - study results and methodological reflections.BMC Med Ethics. 2017 Dec 20;18(1):78. doi: 10.1186/s12910-017-0232-7. BMC Med Ethics. 2017. PMID: 29262814 Free PMC article.
-
The privacy-reciprocity connection in biobanking: comparing German with UK strategies.Public Health Genomics. 2012;15(5):272-84. doi: 10.1159/000336671. Epub 2012 Jun 20. Public Health Genomics. 2012. PMID: 22722691
-
"As Long as You Ask": A Qualitative Study of Biobanking Consent-Oncology Patients' and Health Care Professionals' Attitudes, Motivations, and Experiences-the B-PPAE Study.Oncologist. 2019 Jun;24(6):844-856. doi: 10.1634/theoncologist.2018-0233. Epub 2018 Nov 9. Oncologist. 2019. PMID: 30413662 Free PMC article.
-
Research participants' perceptions and views on consent for biobank research: a review of empirical data and ethical analysis.BMC Med Ethics. 2015 Sep 9;16:60. doi: 10.1186/s12910-015-0053-5. BMC Med Ethics. 2015. PMID: 26354520 Free PMC article. Review.
-
Participants' Understanding of Informed Consent for Biobanking: A Systematic Review.Clin Nurs Res. 2019 Jan;28(1):30-51. doi: 10.1177/1054773817722690. Epub 2017 Jul 26. Clin Nurs Res. 2019. PMID: 28745067
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials