Antibiotic susceptibility profiles of Mycoplasma synoviae strains originating from Central and Eastern Europe
- PMID: 29149886
- PMCID: PMC5693497
- DOI: 10.1186/s12917-017-1266-2
Antibiotic susceptibility profiles of Mycoplasma synoviae strains originating from Central and Eastern Europe
Abstract
Background: Mycoplasma synoviae causes infectious synovitis and respiratory diseases in chickens and turkeys and may lead to egg shell apex abnormalities in chickens; hence possesses high economic impact on the poultry industry. Control of the disease consists of eradication, vaccination or medication. The aim of the present study was to determine the in vitro susceptibility to 14 different antibiotics and an antibiotic combination of M. synoviae strains originating from Hungary and other countries of Central and Eastern Europe.
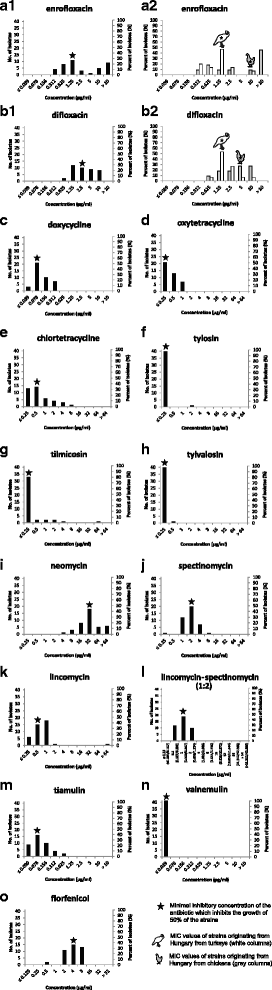
Results: Minimal inhibitory concentration (MIC) values of a total of 41 M. synoviae strains were determined by the microbroth dilution method. The strains were collected between 2002 and 2016 and originated from Hungary (n = 26), Austria (n = 3), the Czech Republic (n = 3), Slovenia (n = 3), Ukraine (n = 3), Russia (n = 2) and Serbia (n = 1). Tetracyclines (with MIC50 values of 0.078 μg/ml, ≤0.25 μg/ml and 0.5 μg/ml for doxycycline, oxytetracycline and chlortetracycline, respectively), macrolides (with MIC50 values of ≤0.25 μg/ml for tylvalosin, tylosin and tilmicosin), pleuromutilins (with MIC50 values of 0.078 μg/ml and ≤0.039 μg/ml for tiamulin and valnemulin) and the combination of lincomycin and spectinomycin (MIC50 1 μg/ml (0.333/0.667 μg/ml)) were found to be the most effective antibiotic agents against M. synoviae in vitro. High MIC values were detected in numerous strains for fluoroquinolones (with MIC50 values of 1.25 μg/ml and 2.5 μg/ml for enrofloxacin and difloxacin), neomycin (MIC50 32 μg/ml), spectinomycin (MIC50 2 μg/ml), lincomycin (MIC50 0.5 μg/ml) and florfenicol (MIC50 4 μg/ml). Nevertheless, strains with elevated MIC values were detected for most of the applied antibiotics.
Conclusions: In the medical control of M. synoviae infections the preliminary in vitro antibiotic susceptibility testing and the careful evaluation of the data are crucial. Based on the in vitro examinations doxycycline, oxytetracycline, tylvalosin, tylosin and pleuromutilins could be recommended for the therapy of M. synoviae infections in the region.
Keywords: Antibiotic resistance; Chicken; MIC; Microbroth dilution; Mycoplasma synoviae; Turkey.
Conflict of interest statement
Ethics approval and consent to participate
All samples were collected by the authors during routine diagnostic examinations or necropsies with the consent of the owners, thus ethical approval or specific permission was not required for the study. Consent to participate is not applicable.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

