Detection of Plasmodium falciparum male and female gametocytes and determination of parasite sex ratio in human endemic populations by novel, cheap and robust RTqPCR assays
- PMID: 29149898
- PMCID: PMC5693539
- DOI: 10.1186/s12936-017-2118-z
Detection of Plasmodium falciparum male and female gametocytes and determination of parasite sex ratio in human endemic populations by novel, cheap and robust RTqPCR assays
Abstract
Background: The presence of Plasmodium falciparum gametocytes in peripheral blood is essential for human to mosquito parasite transmission. The detection of submicroscopic infections with gametocytes and the estimation of the gametocyte sex ratio are crucial to assess the human host potential ability to infect mosquitoes and transmit malaria parasites.
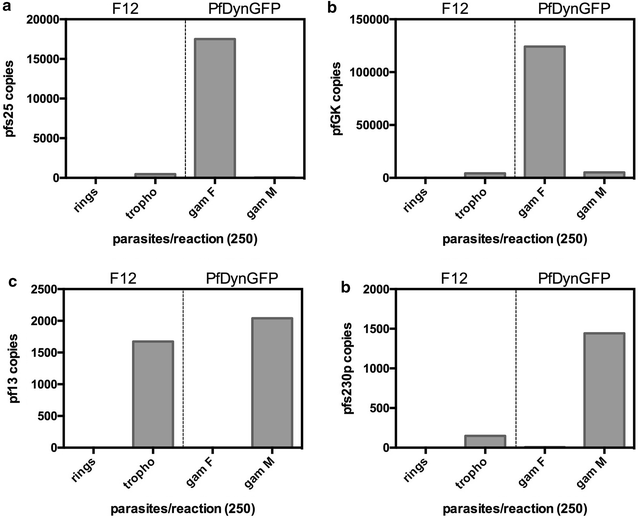
Aim and objectives: The aim of this work was to develop sensitive and cheap Real Time qPCR assays for large-scale epidemiological surveys, based on detection and amplification of gametocyte sex specific transcripts selected from the literature: the female-specific pfs25 and pf glycerol kinase (pfGK) and the male-specific pfs230p and pf13 transcripts.
Methods: RTqPCR assays were used to test the gametocyte- and sex-specific expression of the target genes using asexual stages of the gametocyteless parasite clone F12 and FACS purified male and female gametocytes of the PfDynGFP/P47mCherry line. Assays were performed on 50 blood samples collected during an epidemiological survey in the Soumousso village, Burkina Faso, West-Africa, and amplification of the human housekeeping gene 18S rRNA was employed to normalize RNA sample variability.
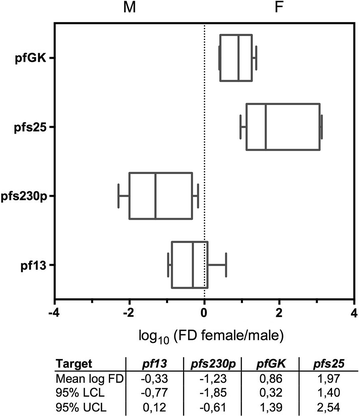
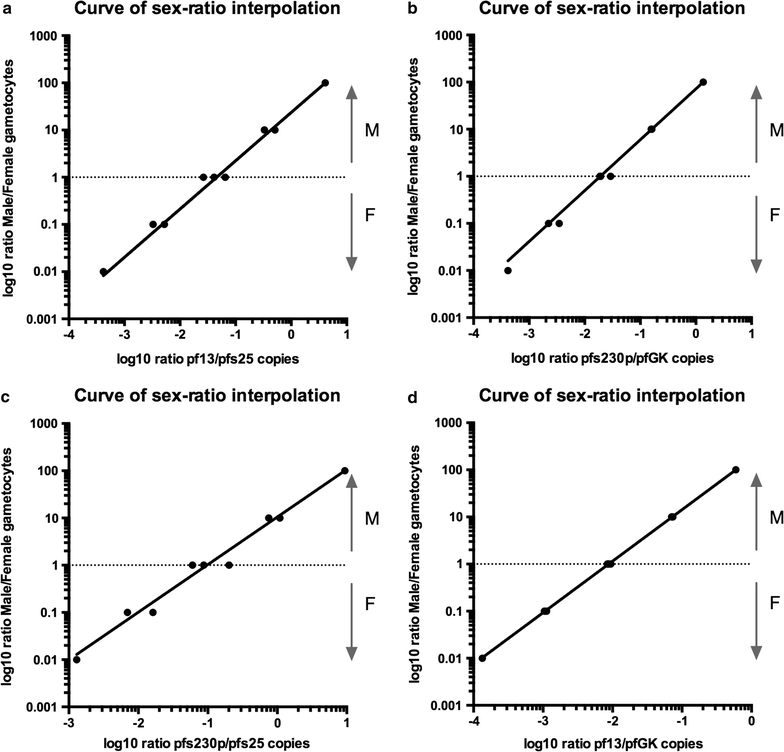
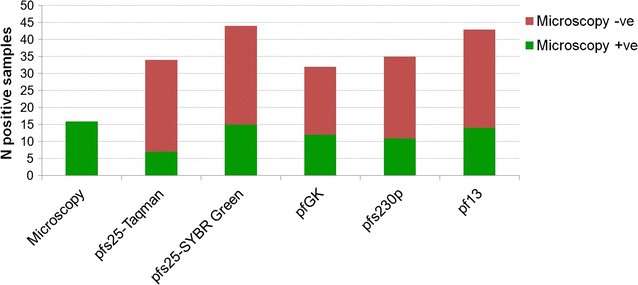
Results: SYBR Green assays were developed that showed higher sensitivity compared to Taqman assays at a reduced cost. RTqPCR results confirmed that expression of pfs25 and pfs230p are female and male-specific, respectively, and introduced two novel markers, the female-specific pfGK and the male-specific pf13. A formula was derived to calculate the ratio of male to female gametocytes based on the ratio of male to female transcript copy number. Use of these assays in the field samples showed, as expected, a higher sensitivity of RTqPCR compared to microscopy. Importantly, similar values of gametocyte sex-ratio were obtained in the field samples based on the four different target combinations.
Conclusion: Novel, sensitive, cheap and robust molecular assays were developed for the detection and quantification of female and male P. falciparum gametocytes. In particular, the RTqPCR assays based on the female-specific pfs25 and the newly described male gametocyte-specific pf13 transcripts, including normalization by the human 18S, reliably assess presence and abundance of female and male gametocytes and enable to determine their sex-ratio in human subjects in endemic areas.
Keywords: Gametocytes; Malaria transmission; Plasmodium falciparum; Real Time qPCR; Sex-ratio.
Figures
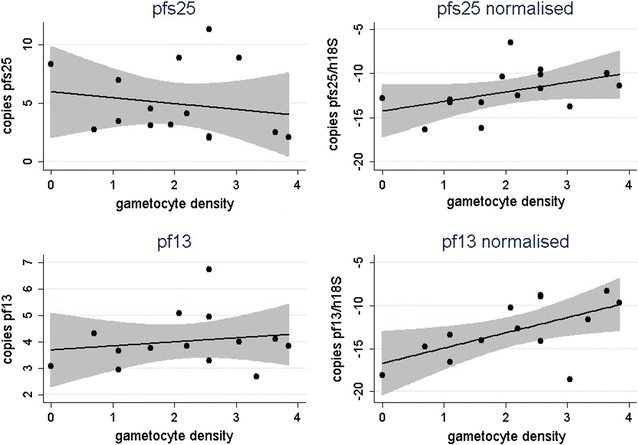
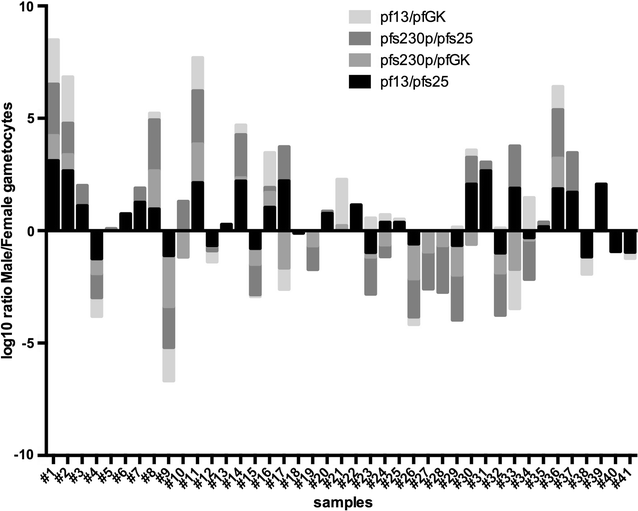
Similar articles
-
Assays for quantification of male and female gametocytes in human blood by qRT-PCR in the absence of pure sex-specific gametocyte standards.Malar J. 2020 Jun 23;19(1):218. doi: 10.1186/s12936-020-03291-9. Malar J. 2020. PMID: 32576184 Free PMC article.
-
Dynamics of Plasmodium falciparum gametocyte carriage in pregnant women under intermittent preventive treatment with sulfadoxine-pyrimethamine in Benin.Malar J. 2018 Oct 11;17(1):356. doi: 10.1186/s12936-018-2498-8. Malar J. 2018. PMID: 30305101 Free PMC article.
-
Quantification of female and male Plasmodium falciparum gametocytes by reverse transcriptase quantitative PCR.Mol Biochem Parasitol. 2015 Jan-Feb;199(1-2):29-33. doi: 10.1016/j.molbiopara.2015.03.006. Epub 2015 Mar 28. Mol Biochem Parasitol. 2015. PMID: 25827756
-
Molecular approaches to monitor parasite genetic complexity in the transmission of Plasmodium falciparum malaria.Parassitologia. 2005 Jun;47(2):199-203. Parassitologia. 2005. PMID: 16252474 Review.
-
Application of molecular methods for monitoring transmission stages of malaria parasites.Biomed Mater. 2008 Sep;3(3):034007. doi: 10.1088/1748-6041/3/3/034007. Epub 2008 Aug 15. Biomed Mater. 2008. PMID: 18708712 Review.
Cited by
-
Assays for quantification of male and female gametocytes in human blood by qRT-PCR in the absence of pure sex-specific gametocyte standards.Malar J. 2020 Jun 23;19(1):218. doi: 10.1186/s12936-020-03291-9. Malar J. 2020. PMID: 32576184 Free PMC article.
-
Biology and epidemiology of Plasmodium falciparum and Plasmodium vivax gametocyte carriage: Implication for malaria control and elimination.Parasite Epidemiol Control. 2023 Mar 7;21:e00295. doi: 10.1016/j.parepi.2023.e00295. eCollection 2023 May. Parasite Epidemiol Control. 2023. PMID: 36950502 Free PMC article. Review.
-
Dynamics of Plasmodium falciparum gametocyte carriage in pregnant women under intermittent preventive treatment with sulfadoxine-pyrimethamine in Benin.Malar J. 2018 Oct 11;17(1):356. doi: 10.1186/s12936-018-2498-8. Malar J. 2018. PMID: 30305101 Free PMC article.
-
A multiplex assay for the sensitive detection and quantification of male and female Plasmodium falciparum gametocytes.Malar J. 2018 Nov 29;17(1):441. doi: 10.1186/s12936-018-2584-y. Malar J. 2018. PMID: 30497508 Free PMC article.
-
Plasmodium falciparum sexual differentiation in malaria patients is associated with host factors and GDV1-dependent genes.Nat Commun. 2019 May 13;10(1):2140. doi: 10.1038/s41467-019-10172-6. Nat Commun. 2019. PMID: 31086187 Free PMC article.
References
-
- WHO. World Malaria Report 2016. Geneva: World Health Organization; 2016. http://www.who.int/malaria/publications/world-malaria-report-2016/report....
-
- Eichner M, Diebner HH, Molineaux L, Collins WE, Jeffery GM, Dietz K. Genesis, sequestration and survival of Plasmodium falciparum gametocytes: parameter estimates from fitting a model to malaria therapy data. Trans R Soc Trop Med Hyg. 2001;95:497–501. doi: 10.1016/S0035-9203(01)90016-1. - DOI - PubMed
-
- Bousema T, Okell L, Shekalaghe S, Griffin JT, Omar S, Sawa P, et al. Revisiting the circulation time of Plasmodium falciparum gametocytes: molecular detection methods to estimate the duration of gametocyte carriage and the effect of gametocytocidal drugs. Malar J. 2010;9:136. doi: 10.1186/1475-2875-9-136. - DOI - PMC - PubMed
-
- Schneider P, Bousema JT, Gouagna LC, Otieno S, van de Vegte-Bolmer M, Omar SA, et al. Submicroscopic Plasmodium falciparum gametocyte densities frequently result in mosquito infection. Am J Trop Med Hyg. 2007;76:470–474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

