Concomitant, sequential, and 7-day triple therapy in first-line treatment of Helicobacter pylori infection in Korea: study protocol for a randomized controlled trial
- PMID: 29149904
- PMCID: PMC5693582
- DOI: 10.1186/s13063-017-2281-0
Concomitant, sequential, and 7-day triple therapy in first-line treatment of Helicobacter pylori infection in Korea: study protocol for a randomized controlled trial
Erratum in
-
Correction to: Concomitant, sequential, and 7-day triple therapy in first-line treatment of Helicobacter pylori infection in Korea: study protocol for a randomized controlled trial.Trials. 2017 Dec 15;18(1):599. doi: 10.1186/s13063-017-2393-6. Trials. 2017. PMID: 29246171 Free PMC article.
Abstract
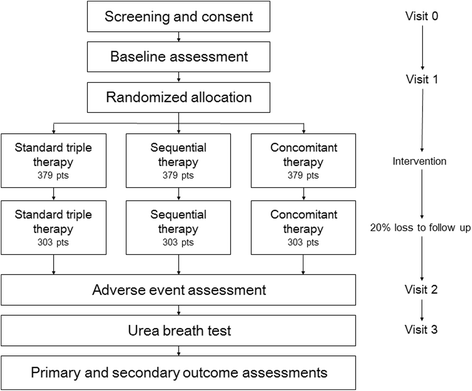
Background: Most international guidelines recommend triple-therapy regimens consisting of a proton pump inhibitor, clarithromycin, and amoxicillin/metronidazole for at least 7 days for the eradication of Helicobacter pylori. However, the efficacy of 7-day clarithromycin-based standard triple therapy for H. pylori infection is currently unacceptable in Korea. In this study, we will compare the efficacy and safety of 7-day standard triple therapy, 10-day sequential therapy, and 10-day concomitant therapy for the first-line treatment of H. pylori infection in Korea.
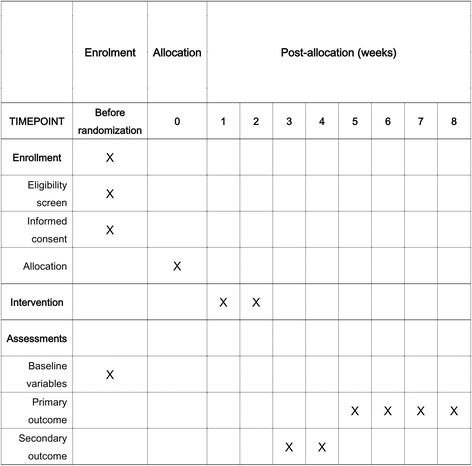
Methods/design: In this multicenter, investigator-blinded, randomized trial we are recruiting adult patients with H. pylori infection from 15 hospitals in Korea to determine whether sequential or concomitant treatment is superior to standard triple therapy. Patients are randomly assigned to receive either standard triple therapy (lansoprazole, amoxicillin, and clarithromycin) for 7 days, or sequential treatment (lansoprazole and amoxicillin for the first 5 days, followed by lansoprazole, clarithromycin, and metronidazole for another 5 days) for 10 days, or concomitant therapy (lansoprazole, amoxicillin, clarithromycin, and metronidazole) for 10 days. The primary outcome is the rate of H. pylori eradication in the intention-to-treat population.
Discussion: The results of this study will be crucial for determining the optimal regimen for the primary treatment of H. pylori infection in Korea. This study will produce vital evidence that will lead to revisions to guidelines concerning first-line treatment regimens for H. pylori infection.
Trial registration: Clinical Research Information Service (CRIS), Republic of Korea, KCT0001980 . Registered on 25 July 2016.
Keywords: Concomitant therapy; Helicobacter pylori; Sequential therapy; Triple therapy.
Conflict of interest statement
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Ten-Day Concomitant, 10-Day Sequential, and 7-Day Triple Therapy as First-Line Treatment for Helicobacter pylori Infection: A Nationwide Randomized Trial in Korea.Gut Liver. 2019 Sep 15;13(5):531-540. doi: 10.5009/gnl19136. Gut Liver. 2019. PMID: 31505907 Free PMC article. Clinical Trial.
-
14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial.Lancet. 2011 Aug 6;378(9790):507-14. doi: 10.1016/S0140-6736(11)60825-8. Epub 2011 Jul 21. Lancet. 2011. PMID: 21777974 Free PMC article. Clinical Trial.
-
Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial.Lancet. 2016 Nov 12;388(10058):2355-2365. doi: 10.1016/S0140-6736(16)31409-X. Epub 2016 Oct 18. Lancet. 2016. PMID: 27769562 Clinical Trial.
-
Systematic Review with Meta-Analysis: Concomitant Therapy vs. Triple Therapy for the First-Line Treatment of Helicobacter pylori Infection.Am J Gastroenterol. 2018 Oct;113(10):1444-1457. doi: 10.1038/s41395-018-0217-2. Epub 2018 Aug 31. Am J Gastroenterol. 2018. PMID: 30171216
-
Efficacy of Vonoprazan-Based Triple Therapy for Helicobacter pylori Eradication: A Multicenter Study and a Review of the Literature.Dig Dis Sci. 2017 Nov;62(11):3069-3076. doi: 10.1007/s10620-017-4664-1. Epub 2017 Jun 29. Dig Dis Sci. 2017. PMID: 28664410 Review.
Cited by
-
Ten-Day Concomitant, 10-Day Sequential, and 7-Day Triple Therapy as First-Line Treatment for Helicobacter pylori Infection: A Nationwide Randomized Trial in Korea.Gut Liver. 2019 Sep 15;13(5):531-540. doi: 10.5009/gnl19136. Gut Liver. 2019. PMID: 31505907 Free PMC article. Clinical Trial.
-
Correction to: Concomitant, sequential, and 7-day triple therapy in first-line treatment of Helicobacter pylori infection in Korea: study protocol for a randomized controlled trial.Trials. 2017 Dec 15;18(1):599. doi: 10.1186/s13063-017-2393-6. Trials. 2017. PMID: 29246171 Free PMC article.
-
Use of eradication therapy in adjunction to periodontal therapy versus alone for treatment of Helicobacter pylori infections: a mini review.Ann Med Surg (Lond). 2023 May 3;85(6):2756-2760. doi: 10.1097/MS9.0000000000000741. eCollection 2023 Jun. Ann Med Surg (Lond). 2023. PMID: 37363585 Free PMC article.
-
Two-week bismuth-containing quadruple therapy and concomitant therapy are effective first-line treatments for Helicobacter pylori eradication: A prospective open-label randomized trial.World J Gastroenterol. 2019 Dec 14;25(46):6790-6798. doi: 10.3748/wjg.v25.i46.6790. World J Gastroenterol. 2019. PMID: 31857780 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical