The Kusamala Program for primary caregivers of children 6-59 months of age hospitalized with severe acute malnutrition in Malawi: study protocol for a cluster-randomized controlled trial
- PMID: 29149905
- PMCID: PMC5693531
- DOI: 10.1186/s13063-017-2299-3
The Kusamala Program for primary caregivers of children 6-59 months of age hospitalized with severe acute malnutrition in Malawi: study protocol for a cluster-randomized controlled trial
Abstract
Background: Severe acute malnutrition (SAM) is associated with high mortality rates and impairments in growth and development in children that do survive. There are complex nutritional, health, and behavioural risk factors involving severely malnourished children and their primary caregivers, requiring integrated intervention approaches.
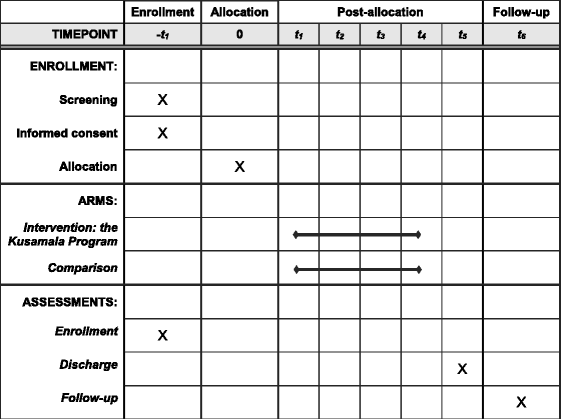
Methods: A cluster-randomized controlled trial at the Queen Elizabeth Central Hospital in Blantyre, Malawi will be conducted to evaluate the effectiveness of a 4-day hospital-based intervention programme directed at primary caregivers. This programme, titled the Kusamala Program, aims to improve developmental and nutritional outcomes in children with SAM. Up to six primary caregivers and their children will be enrolled to groups each week, which will be randomly allocated to intervention or comparison arms. The intervention package consists of interactive counselling on three modules: 1) nutrition and feeding; 2) water, sanitation, and hygiene (WASH); and 3) psychosocial stimulation. Data collection will be performed at enrolment, at discharge from hospital, and at 6 months following discharge. The primary outcome is child development assessed with the Malawi Developmental Assessment Tool (MDAT), a validated measure of gross and fine motor, language, and social development.
Discussion: This intervention programme is unique because it utilizes primary caregivers' time spent in-hospital while children receive treatment for SAM. The programme has the potential to be effective in addressing multiple aspects of child, nutrition and development.
Trial registration: ClinicalTrials.gov, NCT03072433 . Registered on 7 March 2017.
Keywords: Child development; Nutrition; Psychosocial stimulation; RCT; Severe acute malnutrition; WASH.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval for this study was obtained from the College of Medicine Research and Ethics Committee (P.04/16/1930) in Blantyre, Malawi, and the Research Ethics Board (1000053578) at the Hospital for Sick Children in Toronto, Canada. Consent will be requested of all participants in the study; primary caregivers will be asked to provide consent on behalf of themselves and their children.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- World Health Organization. WHO child growth standards and the identification of severe acute malnutrition in infants and children. Geneva: World Health Organization. 2009. Available: http://www.who.int/nutrition/publications/severemalnutrition/97892415981.... Accessed 31 July 2017. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical