Testing the effectiveness of a motivational interviewing-based brief intervention for substance use as an adjunct to usual care in community-based AIDS service organizations: study protocol for a multisite randomized controlled trial
- PMID: 29149914
- PMCID: PMC5693500
- DOI: 10.1186/s13722-017-0095-8
Testing the effectiveness of a motivational interviewing-based brief intervention for substance use as an adjunct to usual care in community-based AIDS service organizations: study protocol for a multisite randomized controlled trial
Erratum in
-
Correction to: Testing the effectiveness of a motivational interviewing-based brief intervention for substance use as an adjunct to usual care in community-based AIDS service organizations: study protocol for a multisite randomized controlled trial.Addict Sci Clin Pract. 2018 Feb 21;13(1):9. doi: 10.1186/s13722-018-0107-3. Addict Sci Clin Pract. 2018. PMID: 29467033 Free PMC article.
Abstract
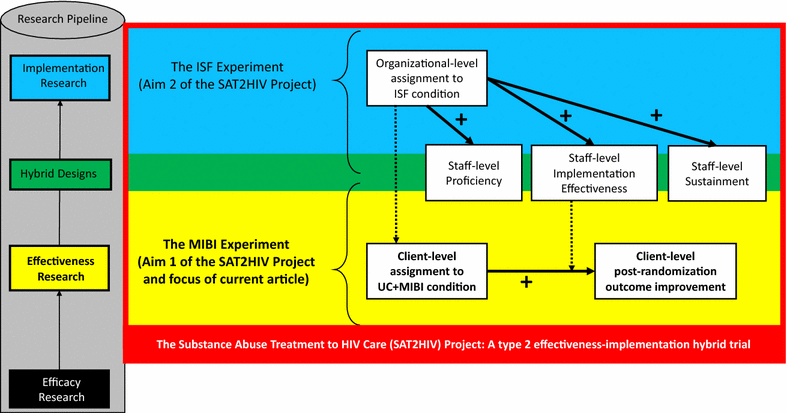
Background: In 2010, the first comprehensive National HIV/AIDS Strategy for the United States was released and included three goals: (1) reducing the number of people who become infected with HIV, (2) increasing access to care and improving health outcomes for people living with HIV, and (3) reducing HIV-related health disparities and health inequities. In 2013, as part of its effort to help address the National HIV/AIDS Strategy, the National Institute on Drug Abuse (NIDA) funded a type 2 effectiveness-implementation hybrid trial titled the Substance Abuse Treatment to HIV Care (SAT2HIV) Project. Aim 1 of the SAT2HIV Project tests the effectiveness of a motivational interviewing-based brief intervention (MIBI) for substance use as an adjunct to usual care within AIDS Service Organizations (ASOs) as part of its MIBI Experiment. Aim 2 of the SAT2HIV Project tests the effectiveness of implementation and sustainment facilitation (ISF) as an adjunct to the Addiction Technology Transfer Center (ATTC) model for training staff in motivational interviewing as part of its ISF Experiment. The current paper describes the study protocol for the MIBI Experiment.
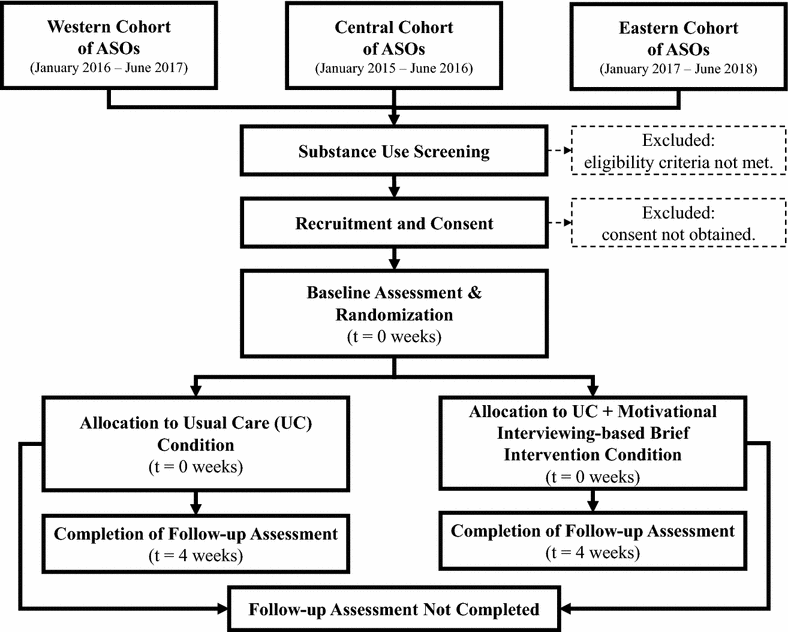
Methods: As part of a multisite randomized controlled trial, individuals with comorbid HIV/AIDS and problematic substance use are randomized to receive either the ASOs' usual care (control condition) or usual care plus a MIBI for substance use (experimental condition) delivered by trained ASO case-management staff. Primary outcome measures are reductions in days of primary substance use, number of substance-related problems, times engaging in risky behaviors, days of non-adherence to HIV medications, and increases in substance use treatment. As part of this paper, we describe the trial protocol in accordance with the Standard Protocol Items: Recommendations for Interventional Trials guidelines.
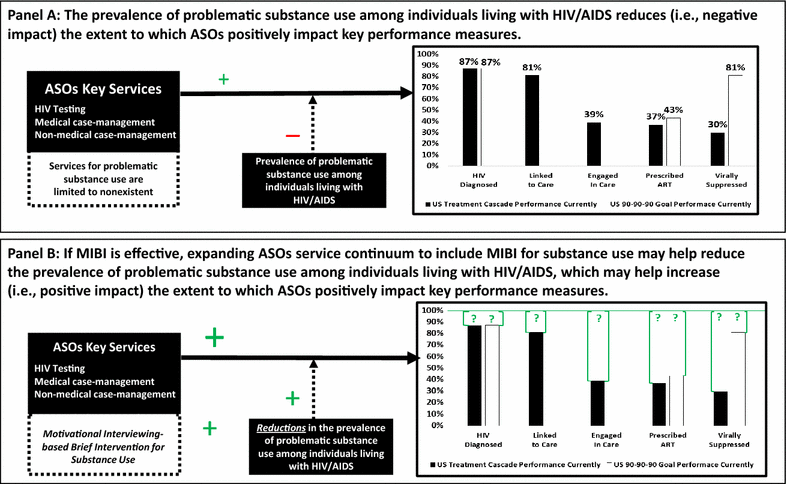
Discussion: If successfully able to implement MIBI as an effective adjunct to usual care, the current trial may have a significant impact on increasing the capacity of ASOs to address problematic substance use among individuals living with HIV/AIDS. Reducing the prevalence of problematic substance use among individuals living with HIV/AIDS within the United States may lead to significant improvements on key performance measures (i.e., the HIV Care Continuum and the 90-90-90 target). Trial registration ClinicalTrials.gov: NCT02495402.
Keywords: AIDS; HIV; Substance use; type 2 hybrid trial.
Figures

References
-
- White House Office of National AIDS Policy. National HIV/AIDS strategy for the United States. Updated to 2020. https://www.aids.gov/federal-resources/national-hiv-aids-strategy/nhas-u... (2015). Accessed 28 Aug 2015.
-
- Centers for Disease Control and Prevention. New HIV infections in the United States. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/new-hiv-infections-... (2016). Accessed 10 Jan 2017.
-
- Centers for Disease Control and Prevention. HIV cost-effectiveness. http://www.cdc.gov/hiv/prevention/ongoing/costeffectiveness/ (2013). Accessed 28 Aug 2015.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

