Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial
- PMID: 29150198
- PMCID: PMC5806119
- DOI: 10.1016/S0140-6736(17)32812-X
Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial
Abstract
Background: Extended-release naltrexone (XR-NTX), an opioid antagonist, and sublingual buprenorphine-naloxone (BUP-NX), a partial opioid agonist, are pharmacologically and conceptually distinct interventions to prevent opioid relapse. We aimed to estimate the difference in opioid relapse-free survival between XR-NTX and BUP-NX.
Methods: We initiated this 24 week, open-label, randomised controlled, comparative effectiveness trial at eight US community-based inpatient services and followed up participants as outpatients. Participants were 18 years or older, had Diagnostic and Statistical Manual of Mental Disorders-5 opioid use disorder, and had used non-prescribed opioids in the past 30 days. We stratified participants by treatment site and opioid use severity and used a web-based permuted block design with random equally weighted block sizes of four and six for randomisation (1:1) to receive XR-NTX or BUP-NX. XR-NTX was monthly intramuscular injections (Vivitrol; Alkermes) and BUP-NX was daily self-administered buprenorphine-naloxone sublingual film (Suboxone; Indivior). The primary outcome was opioid relapse-free survival during 24 weeks of outpatient treatment. Relapse was 4 consecutive weeks of any non-study opioid use by urine toxicology or self-report, or 7 consecutive days of self-reported use. This trial is registered with ClinicalTrials.gov, NCT02032433.
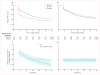
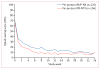
Findings: Between Jan 30, 2014, and May 25, 2016, we randomly assigned 570 participants to receive XR-NTX (n=283) or BUP-NX (n=287). The last follow-up visit was Jan 31, 2017. As expected, XR-NTX had a substantial induction hurdle: fewer participants successfully initiated XR-NTX (204 [72%] of 283) than BUP-NX (270 [94%] of 287; p<0·0001). Among all participants who were randomly assigned (intention-to-treat population, n=570) 24 week relapse events were greater for XR-NTX (185 [65%] of 283) than for BUP-NX (163 [57%] of 287; hazard ratio [HR] 1·36, 95% CI 1·10-1·68), most or all of this difference accounted for by early relapse in nearly all (70 [89%] of 79) XR-NTX induction failures. Among participants successfully inducted (per-protocol population, n=474), 24 week relapse events were similar across study groups (p=0·44). Opioid-negative urine samples (p<0·0001) and opioid-abstinent days (p<0·0001) favoured BUP-NX compared with XR-NTX among the intention-to-treat population, but were similar across study groups among the per-protocol population. Self-reported opioid craving was initially less with XR-NTX than with BUP-NX (p=0·0012), then converged by week 24 (p=0·20). With the exception of mild-to-moderate XR-NTX injection site reactions, treatment-emergent adverse events including overdose did not differ between treatment groups. Five fatal overdoses occurred (two in the XR-NTX group and three in the BUP-NX group).
Interpretation: In this population it is more difficult to initiate patients to XR-NTX than BUP-NX, and this negatively affected overall relapse. However, once initiated, both medications were equally safe and effective. Future work should focus on facilitating induction to XR-NTX and on improving treatment retention for both medications.
Funding: NIDA Clinical Trials Network.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
All authors report grant or contract funding from the National Institute of Drug Abuse (NIDA) for this study. JDL and JR received other research support from NIDA and National Institute on Alcohol Abuse and in-kind study drug from Alkermes for another trial. EVN Jr received other research support from NIDA, Brainsway, Braeburn Pharma, and Alkermes, unpaid consulting fees from Alkermes, and consulting fees from the University of Arkansas. MF received other research support from Alkermes, US World Meds, MediaRez, and the Laura and John Arnold Foundation, and consulting fees from Alkermes and US World Meds. SR received other research support from NIDA, the Heffter Research Institute, Council on Spiritual Practices, and the Sarlo Foundation, and travel support from the Multidisciplinary Association for Psychedelic Studies. All other authors declare no competing interests.
Figures

Comment in
-
Medications for opioid use disorder: bridging the gap in care.Lancet. 2018 Jan 27;391(10118):285-287. doi: 10.1016/S0140-6736(17)32893-3. Epub 2017 Nov 14. Lancet. 2018. PMID: 29150199 No abstract available.
-
Extended-release naltrexone: good but not a panacea.Lancet. 2018 Jan 27;391(10118):283-284. doi: 10.1016/S0140-6736(17)32872-6. Epub 2017 Nov 14. Lancet. 2018. PMID: 29150200 No abstract available.
-
Misclassification of overdose events in the X:BOT study.Lancet. 2023 Aug 12;402(10401):526-527. doi: 10.1016/S0140-6736(23)00113-7. Epub 2023 Jul 19. Lancet. 2023. PMID: 37480936 No abstract available.
References
-
- Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–13. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 DA013046/DA/NIDA NIH HHS/United States
- UG1 DA013034/DA/NIDA NIH HHS/United States
- HHSN271201200017C/DA/NIDA NIH HHS/United States
- UG1 DA013714/DA/NIDA NIH HHS/United States
- U10 DA013714/DA/NIDA NIH HHS/United States
- UG1 DA013035/DA/NIDA NIH HHS/United States
- K24 DA022412/DA/NIDA NIH HHS/United States
- U10 DA015833/DA/NIDA NIH HHS/United States
- U10 DA013720/DA/NIDA NIH HHS/United States
- U10 DA015831/DA/NIDA NIH HHS/United States
- U10 DA013035/DA/NIDA NIH HHS/United States
- HHSN271201500065C/DA/NIDA NIH HHS/United States
- U10 DA013732/DA/NIDA NIH HHS/United States
- U10 DA013045/DA/NIDA NIH HHS/United States
- U10 DA013034/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

