Biogenesis of the bacterial cbb3 cytochrome c oxidase: Active subcomplexes support a sequential assembly model
- PMID: 29150446
- PMCID: PMC5777255
- DOI: 10.1074/jbc.M117.805184
Biogenesis of the bacterial cbb3 cytochrome c oxidase: Active subcomplexes support a sequential assembly model
Abstract
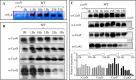
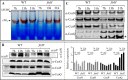
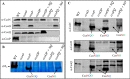
The cbb3 oxidase has a high affinity for oxygen and is required for growth of bacteria, including pathogens, in oxygen-limited environments. However, the assembly of this oxidase is poorly understood. Most cbb3 are composed of four subunits: the catalytic CcoN subunit, the two cytochrome c subunits (CcoO and CcoP) involved in electron transfer, and the small CcoQ subunit with an unclear function. Here, we address the role of these four subunits in cbb3 biogenesis in the purple bacterium Rubrivivax gelatinosus Analyses of membrane proteins from different mutants revealed the presence of active CcoNQO and CcoNO subcomplexes and also showed that the CcoP subunit is not essential for their assembly. However, CcoP was required for the oxygen reduction activity in the absence of CcoQ. We also found that CcoQ is dispensable for forming an active CcoNOP subcomplex in membranes. CcoNOP exhibited oxygen reductase activity, indicating that the cofactors (hemes b and copper for CcoN and cytochromes c for CcoO and CcoP) were present within the subunits. Finally, we discovered the presence of a CcoNQ subcomplex and showed that CcoN is the required anchor for the assembly of the full CcoNQOP complex. On the basis of these findings, we propose a sequential assembly model in which the CcoQ subunit is required for the early maturation step: CcoQ first associates with CcoN before the CcoNQ-CcoO interaction. CcoP associates to CcoNQO subcomplex in the late maturation step, and once the CcoNQOP complex is fully formed, CcoQ is released for degradation by the FtsH protease. This model could be conserved in other bacteria, including the pathogenic bacteria lacking the assembly factor CcoH as in R. gelatinosus.
Keywords: bacteria; cbb3 cytochrome c oxidase biogenesis; cytochrome oxidase; membrane protein; membrane sub-complexes assembly; protein assembly; respiration; subunit interactions.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

