Sulfolobus acidocaldarius Transports Pentoses via a Carbohydrate Uptake Transporter 2 (CUT2)-Type ABC Transporter and Metabolizes Them through the Aldolase-Independent Weimberg Pathway
- PMID: 29150511
- PMCID: PMC5772230
- DOI: 10.1128/AEM.01273-17
Sulfolobus acidocaldarius Transports Pentoses via a Carbohydrate Uptake Transporter 2 (CUT2)-Type ABC Transporter and Metabolizes Them through the Aldolase-Independent Weimberg Pathway
Abstract
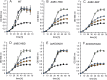
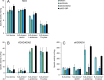
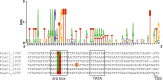
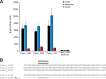
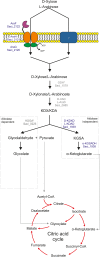
Sulfolobus spp. possess a great metabolic versatility and grow heterotrophically on various carbon sources, such as different sugars and peptides. Known sugar transporters in Archaea predominantly belong to ABC transport systems. Although several ABC transporters for sugar uptake have been characterized in the crenarchaeon Sulfolobus solfataricus, only one homologue of these transporters, the maltose/maltooligomer transporter, could be identified in the closely related Sulfolobus acidocaldarius Comparison of the transcriptome of S. acidocaldarius MW001 grown on peptides alone and peptides in the presence of d-xylose allowed for the identification of the ABC transporter for d-xylose and l-arabinose transport and the gaining of deeper insights into pentose catabolism under the respective growth conditions. The d-xylose/l-arabinose substrate binding protein (SBP) (Saci_2122) of the ABC transporter is unique in Archaea and shares more similarity to bacterial SBPs of the carbohydrate uptake transporter-2 (CUT2) family than to any characterized archaeal one. The identified pentose transporter is the first CUT2 family ABC transporter analyzed in the domain of Archaea Single-gene deletion mutants of the ABC transporter subunits exemplified the importance of the transport system for d-xylose and l-arabinose uptake. Next to the transporter operon, enzymes of the aldolase-independent pentose catabolism branch were found to be upregulated in N-Z-Amine and d-xylose medium. The α-ketoglutarate semialdehyde dehydrogenase (KGSADH; Saci_1938) seemed not to be essential for growth on pentoses. However, the deletion mutant of the 2-keto-3-deoxyarabinoate/xylonate dehydratase (KDXD [also known as KDAD]; Saci_1939) was no longer able to catabolize d-xylose or l-arabinose, suggesting the absence of the aldolase-dependent branch in S. acidocaldariusIMPORTANCE Thermoacidophilic microorganisms are emerging model organisms for biotechnological applications, as their optimal growth conditions resemble conditions used in certain biotechnologies such as industrial plant waste degradation. Because of its high genome stability, Sulfolobus acidocaldarius is especially suited as a platform organism for such applications. For use in (ligno)cellulose degradation, it was important to understand pentose uptake and metabolism in S. acidocaldarius This study revealed that only the aldolase-independent Weimberg pathway is required for growth of S. acidocaldarius MW001 on d-xylose and l-arabinose. Moreover, S. acidocaldarius employs a CUT2 ABC transporter for pentose uptake, which is more similar to bacterial than to archaeal ABC transporters. The identification of pentose-inducible promoters will expedite the metabolic engineering of S. acidocaldarius for its development into a platform organism for (ligno)cellulose degradation.
Keywords: ABC transporters; Sulfolobus carbon metabolism; archaea; pentose metabolism; promoters; sugar transport.
Copyright © 2018 American Society for Microbiology.
Figures
References
-
- Pickl A, Johnsen U, Schönheit P. 2012. Fructose degradation in the haloarchaeon Haloferax volcanii involves a bacterial type phosphoenolpyruvate-dependent phosphotransferase system, fructose-1-phosphate kinase, and class II fructose-1,6-bisphosphate aldolase. J Bacteriol 194:3088–3097. doi: 10.1128/JB.00200-12. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

