A Program for Iron Economy during Deficiency Targets Specific Fe Proteins
- PMID: 29150559
- PMCID: PMC5761800
- DOI: 10.1104/pp.17.01497
A Program for Iron Economy during Deficiency Targets Specific Fe Proteins
Abstract
Iron (Fe) is an essential element for plants, utilized in nearly every cellular process. Because the adjustment of uptake under Fe limitation cannot satisfy all demands, plants need to acclimate their physiology and biochemistry, especially in their chloroplasts, which have a high demand for Fe. To investigate if a program exists for the utilization of Fe under deficiency, we analyzed how hydroponically grown Arabidopsis (Arabidopsis thaliana) adjusts its physiology and Fe protein composition in vegetative photosynthetic tissue during Fe deficiency. Fe deficiency first affected photosynthetic electron transport with concomitant reductions in carbon assimilation and biomass production when effects on respiration were not yet significant. Photosynthetic electron transport function and protein levels of Fe-dependent enzymes were fully recovered upon Fe resupply, indicating that the Fe depletion stress did not cause irreversible secondary damage. At the protein level, ferredoxin, the cytochrome-b6f complex, and Fe-containing enzymes of the plastid sulfur assimilation pathway were major targets of Fe deficiency, whereas other Fe-dependent functions were relatively less affected. In coordination, SufA and SufB, two proteins of the plastid Fe-sulfur cofactor assembly pathway, were also diminished early by Fe depletion. Iron depletion reduced mRNA levels for the majority of the affected proteins, indicating that loss of enzyme was not just due to lack of Fe cofactors. SufB and ferredoxin were early targets of transcript down-regulation. The data reveal a hierarchy for Fe utilization in photosynthetic tissue and indicate that a program is in place to acclimate to impending Fe deficiency.
© 2018 American Society of Plant Biologists. All Rights Reserved.
Figures

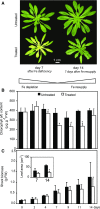
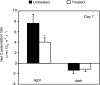
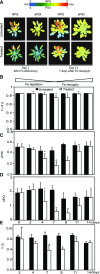

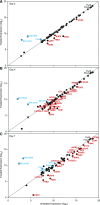
References
-
- Andaluz S, López-Millán A-F, De las Rivas J, Aro EM, Abadía J, Abadía A (2006) Proteomic profiles of thylakoid membranes and changes in response to iron deficiency. Photosynth Res 89: 141–155 - PubMed
-
- Balk J, Pilon M (2011) Ancient and essential: the assembly of iron-sulfur clusters in plants. Trends Plant Sci 16: 218–226 - PubMed
-
- Balk J, Schaedler TA (2014) Iron cofactor assembly in plants. Annu Rev Plant Biol 65: 125–153 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

