Repurposing of the anti-malaria drug chloroquine for Zika Virus treatment and prophylaxis
- PMID: 29150641
- PMCID: PMC5694003
- DOI: 10.1038/s41598-017-15467-6
Repurposing of the anti-malaria drug chloroquine for Zika Virus treatment and prophylaxis
Abstract
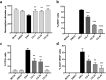
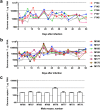
One of the major challenges of the current Zika virus (ZIKV) epidemic is to prevent congenital foetal abnormalities, including microcephaly, following ZIKV infection of pregnant women. Given the urgent need for ZIKV prophylaxis and treatment, repurposing of approved drugs appears to be a viable and immediate solution. We demonstrate that the common anti-malaria drug chloroquine (CQ) extends the lifespan of ZIKV-infected interferon signalling-deficient AG129 mice. However, the severity of ZIKV infection in these mice precludes the study of foetal (vertical) viral transmission. Here, we show that interferon signalling-competent SJL mice support chronic ZIKV infection. Infected dams and sires are both able to transmit ZIKV to the offspring, making this an ideal model for in vivo validation of compounds shown to suppress ZIKV in cell culture. Administration of CQ to ZIKV-infected pregnant SJL mice during mid-late gestation significantly attenuated vertical transmission, reducing the ZIKV load in the foetal brain more than 20-fold. Given the limited side effects of CQ, its lack of contraindications in pregnant women, and its worldwide availability and low cost, we suggest that CQ could be considered for the treatment and prophylaxis of ZIKV.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Li, C. et al. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell, 10.1016/j.stem.2016.04.017 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

