Effect of erythrocyte-sperm separation medium on nuclear, acrosomal, and membrane maturity parameters in human sperm
- PMID: 29150736
- PMCID: PMC5904059
- DOI: 10.1007/s10815-017-1085-1
Effect of erythrocyte-sperm separation medium on nuclear, acrosomal, and membrane maturity parameters in human sperm
Abstract
Purpose: The purpose of this study is to investigate whether erythrocyte-sperm separation medium (ESSM) has effects on human sperm motility, morphology, viability, membrane maturity, acrosome integrity, and nuclear attributes before and after cryopreservation.
Methods: Semen samples from normozoospermic (n = 36) and oligozoospermic (n = 9) patients were analyzed. Samples from the same patient were divided into three aliquots: group 1 and group 2 were resuspended in sperm washing media and ESSM, respectively. Group 3 was resuspended in ESSM with blood sample to mimic the extensive number of erythrocytes in the testicular sperm extraction (TESE) material. All groups were evaluated for sperm concentration, motility, Kruger/Tygerberg strict morphology, viability by eosin-nigrosin staining, membrane maturity by hyaluronic acid-binding assay (HBA), acrosomal integrity by Pisum sativum lectin staining, chromatin maturity by aniline blue staining, and DNA integrity by TUNEL assay before and after cryopreservation.
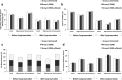
Results: No significant difference was determined between ESSM-treated and ESSM-untreated sperm samples for the sperm parameters tested (p > 0.05). After cryopreservation, total sperm motility and viability decreased regardless of ESSM used. The percentages of sperm with Tygerberg normal morphology, intact acrosome, and HA-bound sperm were found to be lower in oligozoospermic samples before cryopreservation in each group. However, no statistically significant differences were found between oligozoospermic and normozoospermic samples when all groups were compared. Thus, ESSM treatment did not cause a significant change on sperm motility, normal morphology, viability, HA-binding capacity, chromatin maturity, and DNA fragmentation.
Conclusion: ESSM can enhance the efficiency of sperm retrieval protocol and can also decrease the time required to collect spermatozoa while not affecting sperm morphogenetic properties.
Keywords: Cryopreservation; Erythrocyte-sperm separation; Human sperm maturity; Sperm parameters; TESE.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Figures

Similar articles
-
Cryopreservation of Low Number of Human Spermatozoa; Which is Better: Vapor Phase or Direct Submerging in Liquid Nitrogen?Hum Fertil (Camb). 2019 Jun;22(2):126-132. doi: 10.1080/14647273.2018.1456681. Epub 2018 May 11. Hum Fertil (Camb). 2019. PMID: 29749276
-
Canthaxanthin protects human sperm parameters during cryopreservation.Andrologia. 2019 Nov;51(10):e13389. doi: 10.1111/and.13389. Epub 2019 Aug 11. Andrologia. 2019. PMID: 31402476
-
Effects of different cryopreservation methods on DNA integrity and sperm chromatin quality in men.Andrology. 2018 Nov;6(6):829-835. doi: 10.1111/andr.12529. Epub 2018 Sep 2. Andrology. 2018. PMID: 30175448
-
Hyaluronic acid binding by human sperm indicates cellular maturity, viability, and unreacted acrosomal status.Fertil Steril. 2003 Jun;79 Suppl 3:1616-24. doi: 10.1016/s0015-0282(03)00402-3. Fertil Steril. 2003. PMID: 12801568
-
Sperm Separation and Selection Techniques to Mitigate Sperm DNA Damage.Life (Basel). 2025 Feb 14;15(2):302. doi: 10.3390/life15020302. Life (Basel). 2025. PMID: 40003711 Free PMC article. Review.
Cited by
-
Canine and Feline Epididymal Semen-A Plentiful Source of Gametes.Animals (Basel). 2021 Oct 14;11(10):2961. doi: 10.3390/ani11102961. Animals (Basel). 2021. PMID: 34679980 Free PMC article. Review.
-
Sperm Selection Procedures for Optimizing the Outcome of ICSI in Patients with NOA.J Clin Med. 2021 Jun 18;10(12):2687. doi: 10.3390/jcm10122687. J Clin Med. 2021. PMID: 34207121 Free PMC article. Review.
-
Single layer centrifugation combined with brief abstinence for semen cryopreservation in a patient with hematospermia: Case report and literature review.Medicine (Baltimore). 2025 Jul 18;104(29):e43272. doi: 10.1097/MD.0000000000043272. Medicine (Baltimore). 2025. PMID: 40696670 Free PMC article. Review.
-
A comparative study of canine epididymal sperm collection techniques and cryopreservation.Front Vet Sci. 2023 Oct 24;10:1181054. doi: 10.3389/fvets.2023.1181054. eCollection 2023. Front Vet Sci. 2023. PMID: 37954662 Free PMC article.
-
An Alternative Application of Magnetic-Activated Cell Sorting: CD45 and CD235a Based Purification of Semen and Testicular Tissue Samples.Int J Mol Sci. 2024 Mar 24;25(7):3627. doi: 10.3390/ijms25073627. Int J Mol Sci. 2024. PMID: 38612438 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

