GI-530159, a novel, selective, mechanosensitive two-pore-domain potassium (K2P ) channel opener, reduces rat dorsal root ganglion neuron excitability
- PMID: 29150838
- PMCID: PMC5980259
- DOI: 10.1111/bph.14098
GI-530159, a novel, selective, mechanosensitive two-pore-domain potassium (K2P ) channel opener, reduces rat dorsal root ganglion neuron excitability
Abstract
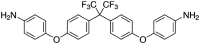
Background and purpose: TREK two-pore-domain potassium (K2P ) channels play a critical role in regulating the excitability of somatosensory nociceptive neurons and are important mediators of pain perception. An understanding of the roles of TREK channels in pain perception and, indeed, in other pathophysiological conditions, has been severely hampered by the lack of potent and/or selective activators and inhibitors. In this study, we describe a new, selective opener of TREK channels, GI-530159.
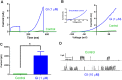
Experimental approach: The effect of GI-530159 on TREK channels was demonstrated using 86 Rb efflux assays, whole-cell and single-channel patch-clamp recordings from recombinant TREK channels. The expression of K2P 2.1 (TREK1), K2P 10.1 (TREK2) and K2P 4.1 (TRAAK) channels was determined using transcriptome analysis from single dorsal root ganglion (DRG) cells. Current-clamp recordings from cultured rat DRG neurons were used to measure the effect of GI-530159 on neuronal excitability.
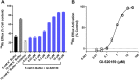
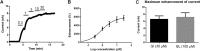
Key results: For recombinant human TREK1 channels, GI-530159 had similar low EC50 values in Rb efflux experiments and electrophysiological recordings. It activated TREK2 channels, but it had no detectable action on TRAAK channels nor any significant effect on other K channels tested. Current-clamp recordings from cultured rat DRG neurones showed that application of GI-530159 at 1 μM resulted in a significant reduction in firing frequency and a small hyperpolarization of resting membrane potential.
Conclusions and implications: This study provides pharmacological evidence for the presence of mechanosensitive TREK K2P channels in sensory neurones and suggests that development of selective K2P channel openers like GI-530159 could aid in the development of novel analgesic agents.
Linked articles: This article is part of a themed section on Recent Advances in Targeting Ion Channels to Treat Chronic Pain. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.12/issuetoc.
© 2017 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

