Efficacy and Safety of Adding Ezetimibe to Statin Therapy Among Women and Men: Insight From IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial)
- PMID: 29151034
- PMCID: PMC5721774
- DOI: 10.1161/JAHA.117.006901
Efficacy and Safety of Adding Ezetimibe to Statin Therapy Among Women and Men: Insight From IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial)
Abstract
Background: IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) showed that adding the nonstatin ezetimibe to statin therapy further reduced cardiovascular events in patients after an acute coronary syndrome. In a prespecified analysis, we explore results stratified by sex.
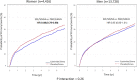
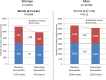
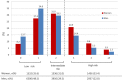
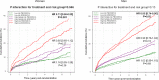
Methods and results: In IMPROVE-IT, patients with acute coronary syndrome and low-density lipoprotein cholesterol of 50 to 125 mg/dL were randomized to placebo/simvastatin 40 mg or ezetimibe/simvastatin 10/40 mg. They were followed up for a median of 6 years for the primary composite of cardiovascular death, myocardial infarction, hospitalization for unstable angina, coronary revascularization ≥30 days, and stroke. Among 18 144 patients in IMPROVE-IT, 4416 (24%) were women. At 12 months, the addition of ezetimibe to simvastatin significantly reduced low-density lipoprotein cholesterol from baseline compared with simvastatin monotherapy in men and women equally (absolute reduction, 16.7 mg/dL in men and 16.4 mg/dL in women). Women receiving ezetimibe/simvastatin had a 12% risk reduction over those receiving placebo/simvastatin for the primary composite end point (hazard ratio, 0.88; 95% confidence interval, 0.79-0.99) compared with a 5% reduction for men (hazard ratio, 0.95; 95% confidence interval, 0.90-1.01; P=0.26 for interaction). When the total number of primary events was considered, women had an 18% reduction with the addition of ezetimibe (relative risk, 95% confidence interval, 0.81; 0.71-0.94) and men had a 6% reduction (relative risk, 0.94; 95% confidence interval, 0.87-1.02; P=0.08 for interaction). The addition of ezetimibe did not increase the rates of safety events in either women or men.
Conclusions: IMPROVE-IT demonstrated that the benefit of adding ezetimibe to statin is present in both women and men, with a good safety profile supporting the use of intensive, combination, lipid-lowering therapy to optimize cardiovascular outcomes.
Clinical trial registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT00202878.
Keywords: cholesterol; chronic ischemic heart disease; coronary artery disease; ezetimibe; lipids and lipoprotein metabolism; secondary prevention; sex; women.
© 2017 The Authors. Published on behalf of the American Heart Association, Inc., by Wiley.
Figures

Similar articles
-
Benefit of Adding Ezetimibe to Statin Therapy on Cardiovascular Outcomes and Safety in Patients With Versus Without Diabetes Mellitus: Results From IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial).Circulation. 2018 Apr 10;137(15):1571-1582. doi: 10.1161/CIRCULATIONAHA.117.030950. Epub 2017 Dec 20. Circulation. 2018. PMID: 29263150 Clinical Trial.
-
Reduction in Total Cardiovascular Events With Ezetimibe/Simvastatin Post-Acute Coronary Syndrome: The IMPROVE-IT Trial.J Am Coll Cardiol. 2016 Feb 2;67(4):353-361. doi: 10.1016/j.jacc.2015.10.077. J Am Coll Cardiol. 2016. PMID: 26821621 Clinical Trial.
-
Prevention of Stroke with the Addition of Ezetimibe to Statin Therapy in Patients With Acute Coronary Syndrome in IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial).Circulation. 2017 Dec 19;136(25):2440-2450. doi: 10.1161/CIRCULATIONAHA.117.029095. Epub 2017 Sep 30. Circulation. 2017. PMID: 28972004 Clinical Trial.
-
Benefit of Combination Ezetimibe/Simvastatin Among High-Risk Populations: Lessons from the IMPROVE-IT Trial.Curr Atheroscler Rep. 2023 Mar;25(3):85-93. doi: 10.1007/s11883-023-01084-4. Epub 2023 Feb 10. Curr Atheroscler Rep. 2023. PMID: 36763180 Review.
-
IMPROVE-IT: what have we learned?Curr Opin Cardiol. 2016 Jul;31(4):426-33. doi: 10.1097/HCO.0000000000000305. Curr Opin Cardiol. 2016. PMID: 27218683 Review.
Cited by
-
Sex specific analysis of patients with and without reported statin intolerance referred to a specialized outpatient lipid clinic.Biol Sex Differ. 2024 Sep 2;15(1):67. doi: 10.1186/s13293-024-00642-y. Biol Sex Differ. 2024. PMID: 39223591 Free PMC article.
-
Pharmacological Strategies beyond Statins: Ezetimibe and PCSK9 Inhibitors.J Lipid Atheroscler. 2019 Sep;8(2):183-191. doi: 10.12997/jla.2019.8.2.183. Epub 2019 Sep 17. J Lipid Atheroscler. 2019. PMID: 32821708 Free PMC article. Review.
-
False versus True Statin Intolerance in Patients with Peripheral Artery Disease.J Clin Med. 2022 Nov 8;11(22):6619. doi: 10.3390/jcm11226619. J Clin Med. 2022. PMID: 36431097 Free PMC article.
-
Unique features of dyslipidemia in women across a lifetime and a tailored approach to management.Am J Prev Cardiol. 2024 Apr 5;18:100666. doi: 10.1016/j.ajpc.2024.100666. eCollection 2024 Jun. Am J Prev Cardiol. 2024. PMID: 38634109 Free PMC article. Review.
-
Impact of Sex Differences on Lipids and Statin Utilization.Curr Atheroscler Rep. 2025 Mar 18;27(1):38. doi: 10.1007/s11883-025-01286-y. Curr Atheroscler Rep. 2025. PMID: 40100457 Review.
References
-
- Gutierrez J, Ramirez G, Rundek T, Sacco RL. Statin therapy in the prevention of recurrent cardiovascular events: a sex‐based meta‐analysis. Arch Intern Med. 2012;172:909–919. - PubMed
-
- Miettinen TA, Pyorala K, Olsson AG, Musliner TA, Cook TJ, Faergeman O, Berg K, Pedersen T, Kjekshus J. Cholesterol‐lowering therapy in women and elderly patients with myocardial infarction or angina pectoris: findings from the Scandinavian Simvastatin Survival Study (4S). Circulation. 1997;96:4211–4218. - PubMed
-
- Kostis WJ, Cheng JQ, Dobrzynski JM, Cabrera J, Kostis JB. Meta‐analysis of statin effects in women versus men. J Am Coll Cardiol. 2012;59:572–582. - PubMed
-
- Lewis SJ, Sacks FM, Mitchell JS, East C, Glasser S, Kell S, Letterer R, Limacher M, Moye LA, Rouleau JL, Pfeffer MA, Braunwald E. Effect of pravastatin on cardiovascular events in women after myocardial infarction: the cholesterol and recurrent events (CARE) trial. J Am Coll Cardiol. 1998;32:140–146. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

