Tenofovir vs lamivudine plus adefovir in chronic hepatitis B: TENOSIMP-B study
- PMID: 29151700
- PMCID: PMC5685852
- DOI: 10.3748/wjg.v23.i41.7459
Tenofovir vs lamivudine plus adefovir in chronic hepatitis B: TENOSIMP-B study
Abstract
Aim: To demonstrate the non-inferiority (15% non-inferiority limit) of monotherapy with tenofovir disoproxil fumarate (TDF) vs the combination of lamivudine (LAM) plus adefovir dipivoxil (ADV) in the maintenance of virologic response in patients with chronic hepatitis B (CHB) and prior failure with LAM.
Methods: This study was a Phase IV prospective, randomized, open, controlled study with 2 parallel groups (TDF and LAM+ADV) of adult patients with hepatitis B e antigen (HBeAg)-negative CHB, prior failure with LAM, on treatment with LAM+ADV for at least 6 mo, without prior resistance to ADV and with an undetectable viral load at the start of the study, in 14 Spanish hospitals. The follow-up time for each patient was 48 wk after randomization, with quarterly visits in which the viral load, biochemical and serological parameters, adverse effects, adherence to treatment and consumption of hospital resources were analysed.
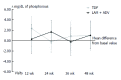
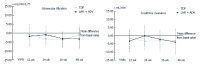
Results: Forty-six patients were evaluated [median age: 55.4 years (30.2-75.2); 84.8% male], including 22 patients with TDF and 24 with LAM+ADV. During study development, hepatitis B virus DNA (HBV-DNA) remained undetectable, all patients remained HBeAg negative, and hepatitis B surface antigen (HBsAg) positive. Alanine aminotransferase (ALT) values at the end of the study were similar in the 2 groups (25.1 ± 7.65, TDF vs 24.22 ± 8.38, LAM+ADV, P = 0.646). No significant changes were observed in creatinine or serum phosphorus values in either group. No significant differences between the 2 groups were noted in the identification of adverse effects (AEs) (53.8%, TDF vs 37.5%, LAM+ADV, P = 0.170), and none of the AEs which occurred were serious. Treatment adherence was 95.5% and 83.3% in the TDF and the LAM+ADV groups, respectively (P = 0.488). The costs associated with hospital resource consumption were significantly lower with the TDF treatment than the LAM+ADV treatment (€4943 ± 1059 vs €5811 ± 1538, respectively, P < 0.001).
Conclusion: TDF monotherapy proved to be safe and not inferior to the LAM+ADV combination therapy in maintaining virologic response in patients with CHB and previous LAM failure. In addition, the use of TDF generated a significant savings in hospital costs.
Keywords: Adherence; Costs; Efficacy; Hepatitis B; Lamivudine+Adefovir; Safety; Tenofovir.
Conflict of interest statement
Conflict-of-interest statement: Manuel Rodríguez consults for and is on the speaker’s bureau for Gilead and AbbVie. Juan Manuel Pascasio consults for and expert advice for BMS, Gilead, AbbVie, MSD and Janssen. Enrique Fraga consults for and is on the speaker’s bureau for Gilead, MSD, BMS and AbbVie. Martín Prieto participates in advisory board for AbbVie, Bristol-Myers, Gilead, Janssen and MSD and in lectures for Bristol-Myers, Gilead, Janssen, Janssen and MSD. José Luis Calleja consults for and is on the speaker’s bureau for: BMS and Gilead. María Luisa Garcia-Buey consults for and is on the speakers´s bureau for AbbVie, Janssen, and Gilead. The remaining authors declare that they have no conflicts of interest.
Figures

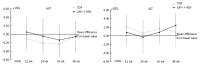
References
-
- Lai CL, Yuen MF. Chronic hepatitis B--new goals, new treatment. N Engl J Med. 2008;359:2488–2491. - PubMed
-
- Feld JJ, Heathcote EJ. Hepatitis B e antigen-positive chronic hepatitis B: natural history and treatment. Semin Liver Dis. 2006;26:116–129. - PubMed
-
- Hadziyannis SJ, Papatheodoridis GV. Hepatitis B e antigen-negative chronic hepatitis B: natural history and treatment. Semin Liver Dis. 2006;26:130–141. - PubMed
-
- Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–2455. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

