Downregulation of lipolysis-stimulated lipoprotein receptor promotes cell invasion via claudin-1-mediated matrix metalloproteinases in human endometrial cancer
- PMID: 29151917
- PMCID: PMC5680700
- DOI: 10.3892/ol.2017.7038
Downregulation of lipolysis-stimulated lipoprotein receptor promotes cell invasion via claudin-1-mediated matrix metalloproteinases in human endometrial cancer
Abstract
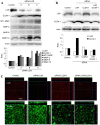
Lipolysis-stimulated lipoprotein receptor (LSR) is a novel molecule present at tricellular contacts which recruits tricellulin (TRIC), a molecular component of tricellular tight junctions (tTJs). LSR and TRIC are colocalized with the bicellular tight junction (bTJ) protein claudin (CLDN)-1-based tight junction strands at tricellular corners. Knockdown of LSR in normal epithelial cells affects tTJ formation and the epithelial barrier function. In cancer cells knockdown of LSR has been demonstrated to increase cell invasion. However, the detailed mechanisms of how the downregulation of LSR enhances cell invasion in cancer remain unclear. In the present study, knockdown of LSR by small interfering RNA (siRNA) in Sawano human endometrial adenocarcinoma cells induced cell invasion. In LSR-knockdown Sawano cells, upregulation of CLDN-1 protein, which contributes to the cell invasion via matrix metalloproteinases (MMPs), was observed compared with the control group by western blotting and immunostaining. Knockdown of LSR significantly induced Sp1 transcription factor activity in the CLDN-1 promoter region. In LSR-knockdown Sawano cells, DNA microarray analysis demonstrated that MMP-1, MMP-2 and MMP-10 mRNA levels were increased, and the protein levels of membrane-type 1-MMP, MMP-2, MMP-9 and MMP-10 were shown to be increased on western blots. Knockdown of CLDN-1 with siRNA prevented the upregulation of cell invasion induced by the knockdown of LSR in Sawano cells. On the invasive front of human endometrial carcinoma tissue samples, a decrease in LSR and increase in CLDN-1 protein levels were observed using immunohistochemical methods. In conclusion, the results indicate that the downregulation of LSR promotes cell invasion of human endometrial cancer via CLDN-1 mediation of MMPs. This mechanism is important for studying the association of tTJs with the cellular invasion of cancer.
Keywords: Sp1 transcription factor; cell invasion; claudin-1; endometrial cancer; lipolysis-stimulated lipoprotein receptor; matrix metalloproteinases.
Figures


Similar articles
-
Loss of tricellular tight junction protein LSR promotes cell invasion and migration via upregulation of TEAD1/AREG in human endometrial cancer.Sci Rep. 2017 Jan 10;7:37049. doi: 10.1038/srep37049. Sci Rep. 2017. PMID: 28071680 Free PMC article.
-
The roles of tricellular tight junction protein lipolysis-stimulated lipoprotein receptor in malignancy of human endometrial cancer cells.Oncotarget. 2016 May 10;7(19):27735-52. doi: 10.18632/oncotarget.8408. Oncotarget. 2016. PMID: 27036040 Free PMC article.
-
The roles of tight junction protein cingulin in human endometrioid endometrial cancer.Tissue Barriers. 2025;13(2):2361976. doi: 10.1080/21688370.2024.2361976. Epub 2024 Jun 2. Tissue Barriers. 2025. PMID: 38825958 Free PMC article.
-
The Roles of Tricellular Tight Junction Protein Angulin-1/Lipolysis-Stimulated Lipoprotein Receptor (LSR) in Endometriosis and Endometrioid-Endometrial Carcinoma.Cancers (Basel). 2021 Dec 17;13(24):6341. doi: 10.3390/cancers13246341. Cancers (Basel). 2021. PMID: 34944960 Free PMC article. Review.
-
Role of Tricellular Tight Junction Protein Lipolysis-Stimulated Lipoprotein Receptor (LSR) in Cancer Cells.Int J Mol Sci. 2019 Jul 20;20(14):3555. doi: 10.3390/ijms20143555. Int J Mol Sci. 2019. PMID: 31330820 Free PMC article. Review.
Cited by
-
Tricellular tight junction protein LSR/angulin-1 contributes to the epithelial barrier and malignancy in human pancreatic cancer cell line.Histochem Cell Biol. 2020 Jan;153(1):5-16. doi: 10.1007/s00418-019-01821-4. Epub 2019 Oct 24. Histochem Cell Biol. 2020. PMID: 31650247
-
Anti-lipolysis-stimulated lipoprotein receptor monoclonal antibody as a novel therapeutic agent for endometrial cancer.BMC Cancer. 2022 Jun 21;22(1):679. doi: 10.1186/s12885-022-09789-6. BMC Cancer. 2022. PMID: 35729527 Free PMC article.
-
Sulforaphane-cysteine inhibited migration and invasion via enhancing mitophagosome fusion to lysosome in human glioblastoma cells.Cell Death Dis. 2020 Oct 1;11(9):819. doi: 10.1038/s41419-020-03024-5. Cell Death Dis. 2020. PMID: 33004792 Free PMC article.
-
Loss of tricellular tight junction protein LSR promotes cell invasion and migration via upregulation of TEAD1/AREG in human endometrial cancer.Sci Rep. 2017 Jan 10;7:37049. doi: 10.1038/srep37049. Sci Rep. 2017. PMID: 28071680 Free PMC article.
-
Downregulation of angulin-1/LSR induces malignancy via upregulation of EGF-dependent claudin-2 and TGF-β-dependent cell metabolism in human lung adenocarcinoma A549 cells.Oncotarget. 2023 Mar 24;14:261-275. doi: 10.18632/oncotarget.27728. Oncotarget. 2023. PMID: 36961882 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
