The Glycolytic Switch in Tumors: How Many Players Are Involved?
- PMID: 29151926
- PMCID: PMC5687156
- DOI: 10.7150/jca.21125
The Glycolytic Switch in Tumors: How Many Players Are Involved?
Abstract
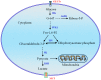
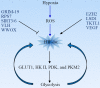
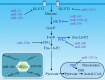
Reprogramming of cellular metabolism is a hallmark of cancers. Cancer cells more readily use glycolysis, an inefficient metabolic pathway for energy metabolism, even when sufficient oxygen is available. This reliance on aerobic glycolysis is called the Warburg effect, and promotes tumorigenesis and malignancy progression. The mechanisms of the glycolytic shift in tumors are not fully understood. Growing evidence demonstrates that many signal molecules, including oncogenes and tumor suppressors, are involved in the process, but how oncogenic signals attenuate mitochondrial function and promote the switch to glycolysis remains unclear. Here, we summarize the current information on several main mediators and discuss their possible mechanisms for triggering the Warburg effect.
Keywords: aerobic glycolysis; glycolytic switch.; reprogramming of glucose metabolism; the Warburg effect; tumor metabolism.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Nolop KB, Rhodes CG, Brudin LH, Beaney RP, Krausz T, Jones T. et al. Glucose utilization in vivo by human pulmonary neoplasms. Cancer. 1987;60:2682–9. - PubMed
-
- Gottschalk S, Anderson N, Hainz C, Eckhardt SG, Serkova NJ. Imatinib (STI571)-mediated changes in glucose metabolism in human leukemia BCR-ABL-positive cells. Clin Cancer Res. 2004;10:6661–8. - PubMed
-
- Martin M, Beauvoit B, Voisin PJ, Canioni P, Guerin B, Rigoulet M. Energetic and morphological plasticity of C6 glioma cells grown on 3-D support; effect of transient glutamine deprivation. J Bioenerg Biomembr. 1998;30:565–78. - PubMed
-
- Pasdois P, Deveaud C, Voisin P, Bouchaud V, Rigoulet M, Beauvoit B. Contribution of the phosphorylable complex I in the growth phase-dependent respiration of C6 glioma cells in vitro. J Bioenerg Biomembr. 2003;35:439–50. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

