Structure-Based Design and Synthesis of New Estrane-Pyridine Derivatives as Cytochrome P450 (CYP) 1B1 Inhibitors
- PMID: 29152048
- PMCID: PMC5683706
- DOI: 10.1021/acsmedchemlett.7b00265
Structure-Based Design and Synthesis of New Estrane-Pyridine Derivatives as Cytochrome P450 (CYP) 1B1 Inhibitors
Abstract
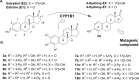
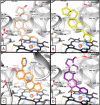
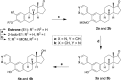
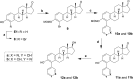
Inhibition of cytochrome P450 (CYP) 1B1 is a promising therapeutic strategy, as such an inhibitor could modulate the bioactivation of procarcinogens while reducing drug resistance. Based on docking studies, the synthesis of 12 estra-1,3,5(10)-triene derivatives containing a pyridin-3-/4-yl moiety at position C2, C3, or C4 was performed, and we measured their inhibitory activity on CYP1B1 using the ethoxyresorufin-O-deethylase (EROD) assay. The position of the nitrogen atom in the aromatic ring has little influence on their inhibition potency, but compounds with a pyridinyl at C2 of the steroid nucleus are more potent CYP1B1 inhibitors than those with a pyridinyl at C3 or C4. Estradiol derivatives (OH at C17β) are also 10-fold more potent inhibitors than estrone derivatives (carbonyl at C17). Thus, 2-(pyridin-3-yl)-estradiol (4a) is the best CYP1B1 inhibitor (IC50 = 0.011 μM) from this series of compounds, and the best steroid inhibitor reported until now. It is also 7.5-fold more potent than the well-known nonsteroidal CYP1B1 inhibitor α-naphthoflavone (IC50 = 0.083 μM).
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Targeting Cytochrome P450 (CYP) 1B1 Enzyme with Four Series of A-Ring Substituted Estrane Derivatives: Design, Synthesis, Inhibitory Activity, and Selectivity.J Med Chem. 2018 Oct 25;61(20):9229-9245. doi: 10.1021/acs.jmedchem.8b00907. Epub 2018 Oct 3. J Med Chem. 2018. PMID: 30216063
-
Targeting cytochrome P450 (CYP) 1B1 with steroid derivatives.Bioorg Med Chem Lett. 2016 Nov 1;26(21):5272-5276. doi: 10.1016/j.bmcl.2016.09.046. Epub 2016 Sep 17. Bioorg Med Chem Lett. 2016. PMID: 27687674
-
Design and Synthesis of New α-Naphthoflavones as Cytochrome P450 (CYP) 1B1 Inhibitors To Overcome Docetaxel-Resistance Associated with CYP1B1 Overexpression.J Med Chem. 2015 Apr 23;58(8):3534-47. doi: 10.1021/acs.jmedchem.5b00265. Epub 2015 Apr 1. J Med Chem. 2015. PMID: 25799264
-
Inhibitors of cytochrome P450 (CYP) 1B1.Eur J Med Chem. 2017 Jul 28;135:296-306. doi: 10.1016/j.ejmech.2017.04.042. Epub 2017 Apr 18. Eur J Med Chem. 2017. PMID: 28458135 Review.
-
Discovery of cytochrome P450 1B1 inhibitors as new promising anti-cancer agents.Med Res Rev. 2003 Nov;23(6):657-68. doi: 10.1002/med.10050. Med Res Rev. 2003. PMID: 12939788 Review.
Cited by
-
Development of benzochalcone derivatives as selective CYP1B1 inhibitors and anticancer agents.Medchemcomm. 2019 Jul 2;10(9):1606-1614. doi: 10.1039/c9md00258h. eCollection 2019 Sep 1. Medchemcomm. 2019. PMID: 31803401 Free PMC article.
-
Synthesis, biological activities, and future perspectives of steroidal monocyclic pyridines.RSC Med Chem. 2023 Sep 7;14(10):1934-1972. doi: 10.1039/d3md00411b. eCollection 2023 Oct 18. RSC Med Chem. 2023. PMID: 37859725 Free PMC article. Review.
-
Computational Approaches in Preclinical Studies on Drug Discovery and Development.Front Chem. 2020 Sep 11;8:726. doi: 10.3389/fchem.2020.00726. eCollection 2020. Front Chem. 2020. PMID: 33062633 Free PMC article. Review.
-
Functionalized Sulfur-Containing Heterocyclic Analogs Induce Sub-G1 Arrest and Apoptotic Cell Death of Laryngeal Carcinoma In Vitro.Molecules. 2023 Feb 15;28(4):1856. doi: 10.3390/molecules28041856. Molecules. 2023. PMID: 36838844 Free PMC article.
-
Recent Advances in Pyridine Scaffold: Focus on Chemistry, Synthesis, and Antibacterial Activities.Biomed Res Int. 2023 May 18;2023:9967591. doi: 10.1155/2023/9967591. eCollection 2023. Biomed Res Int. 2023. PMID: 37250749 Free PMC article. Review.
References
-
- Sutter T. R.; Tang Y. M.; Hayes C. L.; Wo Y. Y.; Jabs E. W.; Li X.; Yin H.; Cody C. W.; Greenlee W. F. Complete cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2. J. Biol. Chem. 1994, 269, 13092–13099. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

