Aspirin exerts high anti-cancer activity in PIK3CA-mutant colon cancer cells
- PMID: 29152088
- PMCID: PMC5675640
- DOI: 10.18632/oncotarget.20972
Aspirin exerts high anti-cancer activity in PIK3CA-mutant colon cancer cells
Abstract
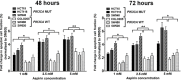
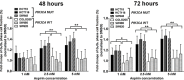
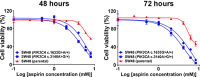
Evidence suggests that nonsteroidal anti-inflammatory drug aspirin (acetylsalicylic acid) may improve patient survival in PIK3CA-mutant colorectal carcinoma, but not in PIK3CA-wild-type carcinoma. However, whether aspirin directly influences the viability of PIK3CA-mutant colon cancer cells is poorly understood. We conducted in vitro experiments to test our hypothesis that the anti-proliferative activity of aspirin might be stronger for PIK3CA-mutant colon cancer cells than for PIK3CA-wild-type colon cancer cells. We measured the anti-proliferative effect of aspirin at physiologic concentrations in seven PIK3CA-mutant and six PIK3CA-wild-type human colon cancer cell lines. After exposure to aspirin, the apoptotic index and cell cycle phase of colon cancer cells were assessed. In addition, the effect of aspirin was examined in parental SW48 cells and SW48 cell clones with individual knock-in PIK3CA mutations of either c.3140A>G (p.H1047R) or c.1633G>A (p.E545K). Aspirin induced greater dose-dependent loss of cell viability in PIK3CA-mutant cells than in PIK3CA-wild-type cells after treatment for 48 and 72 hours. Aspirin treatment also led to higher proportions of apoptotic cells and G0/G1 phase arrest in PIK3CA-mutant cells than in PIK3CA-wild-type cells. Aspirin treatment of isogenic SW48 cells carrying a PIK3CA mutation, either c.3140A>G (p.H1047R) or c.1633G>A (p. E545K), resulted in a more significant loss of cell viability compared to wild-type controls. Our findings indicate that aspirin causes cell cycle arrest, induces apoptosis, and leads to loss of cell viability more profoundly in PIK3CA-mutated colon cancer cells than in PIK3CA-wild-type colon cancer cells. These findings support the use of aspirin to treat patients with PIK3CA-mutant colon cancer.
Keywords: NSAID; PI3K; anti-tumor effect; colorectal cancer; isogenic cell model.
Conflict of interest statement
CONFLICTS OF INTEREST A.T.C. previously served as a consultant for Bayer Healthcare, Pfizer Inc., and Aralez Pharmaceuticals. This study was not funded by Bayer Healthcare, Pfizer Inc. or Aralez Pharmaceuticals. No other conflict of interest exists. The other authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Aspirin sensitivity of PIK3CA-mutated Colorectal Cancer: potential mechanisms revisited.Cell Mol Life Sci. 2022 Jul 2;79(7):393. doi: 10.1007/s00018-022-04430-y. Cell Mol Life Sci. 2022. PMID: 35780223 Free PMC article. Review.
-
Aspirin-Induced Chemoprevention and Response Kinetics Are Enhanced by PIK3CA Mutations in Colorectal Cancer Cells.Cancer Prev Res (Phila). 2017 Mar;10(3):208-218. doi: 10.1158/1940-6207.CAPR-16-0175. Epub 2017 Feb 2. Cancer Prev Res (Phila). 2017. PMID: 28154202 Free PMC article.
-
Inhibitors of STAT3, β-catenin, and IGF-1R sensitize mouse PIK3CA-mutant breast cancer to PI3K inhibitors.Mol Oncol. 2017 May;11(5):552-566. doi: 10.1002/1878-0261.12053. Epub 2017 Apr 6. Mol Oncol. 2017. PMID: 28296140 Free PMC article.
-
Mutations in PIK3CA sensitize breast cancer cells to physiologic levels of aspirin.Breast Cancer Res Treat. 2016 Feb;156(1):33-43. doi: 10.1007/s10549-016-3729-8. Epub 2016 Feb 25. Breast Cancer Res Treat. 2016. PMID: 26915040 Free PMC article.
-
Prognostic and clinical impact of PIK3CA mutation in gastric cancer: pyrosequencing technology and literature review.BMC Cancer. 2016 Jul 7;16:400. doi: 10.1186/s12885-016-2422-y. BMC Cancer. 2016. PMID: 27388016 Free PMC article. Review.
Cited by
-
Colorectal cancer chemoprevention: is aspirin still in the game?Cancer Biol Ther. 2022 Dec 31;23(1):446-461. doi: 10.1080/15384047.2022.2104561. Cancer Biol Ther. 2022. PMID: 35905195 Free PMC article. Review.
-
Aspirin potentiates celecoxib-induced growth inhibition and apoptosis in human non-small cell lung cancer by targeting GRP78 activity.Ther Adv Med Oncol. 2020 Sep 17;12:1758835920947976. doi: 10.1177/1758835920947976. eCollection 2020. Ther Adv Med Oncol. 2020. PMID: 32994805 Free PMC article.
-
Aspirin sensitivity of PIK3CA-mutated Colorectal Cancer: potential mechanisms revisited.Cell Mol Life Sci. 2022 Jul 2;79(7):393. doi: 10.1007/s00018-022-04430-y. Cell Mol Life Sci. 2022. PMID: 35780223 Free PMC article. Review.
-
Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells.Med Sci (Basel). 2018 Apr 13;6(2):31. doi: 10.3390/medsci6020031. Med Sci (Basel). 2018. PMID: 29652830 Free PMC article. Review.
-
Deactivation of Glutaminolysis Sensitizes PIK3CA-Mutated Colorectal Cancer Cells to Aspirin-Induced Growth Inhibition.Cancers (Basel). 2020 Apr 30;12(5):1097. doi: 10.3390/cancers12051097. Cancers (Basel). 2020. PMID: 32365457 Free PMC article.
References
-
- Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–93. - PubMed
-
- Dienstmann R, Salazar R, Tabernero J. Personalizing colon cancer adjuvant therapy: selecting optimal treatments for individual patients. J Clin Oncol. 2015;33:1787–96. - PubMed
-
- Linnekamp JF, Wang X, Medema JP, Vermeulen L. Colorectal cancer heterogeneity and targeted therapy: a case for molecular disease subtypes. Cancer Res. 2015;75:245–9. - PubMed
-
- Kudryavtseva AV, Lipatova AV, Zaretsky AR, Moskalev AA, Fedorova MS, Rasskazova AS, Shibukhova GA, Snezhkina AV, Kaprin AD, Alekseev BY, Dmitriev AA, Krasnov GS. Important molecular genetic markers of colorectal cancer. Oncotarget. 2016;7:53959–83. https://doi.org/10.18632/oncotarget.9796 - DOI - PMC - PubMed
-
- Patil H, Saxena SG, Barrow CJ, Kanwar JR, Kapat A, Kanwar RK. Chasing the personalized medicine dream through biomarker validation in colorectal cancer. Drug Discov Today. 2017;22:111–19. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

