The role of weekly nanoparticle albumin bound paclitaxel monotherapy as second line or later treatment for advanced NSCLC in China
- PMID: 29152093
- PMCID: PMC5675645
- DOI: 10.18632/oncotarget.21103
The role of weekly nanoparticle albumin bound paclitaxel monotherapy as second line or later treatment for advanced NSCLC in China
Abstract
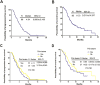
For patients with pretreated advanced non-small cell lung cancer (NSCLC), more effective treatments are unmet. We conducted a study to explore the optimal treatment schedule of nanoparticle albumin bound paclitaxel (Nab-PTX) as a second line or later treatment for advanced NSCLC patients in China. Ninety-eight patients, who had experienced failure of prior treatment and received Nab-PTX monotherapy (130 mg/m2) on days 1, 8 of a 21-day cycle were included. The median progression-free survival (PFS) and overall survival (OS) were 4.34 months (95% confidence interval [CI] 3.508 to 5.165 months) and 11.73 months (95% CI 9.211 to 14.247 months), respectively. The objective responses rate (ORR) and disease control rate (DCR) were 22.4% and 74.5%. Prior treatment with taxane and line of therapy did not influence the efficacy of Nab-PTX. The main grade 3 to 4 toxicities were neutropenia (25.5%) and leukopenia (12.4%). Furthermore, 24 cases offered samples to assess secreted protein acidic and rich in cysteine (SPARC) expression. No statistical difference was observed in treatment efficacy between SPARC expression-negative and positive. The findings suggest that weekly Nab-PTX monotherapy is effective and well tolerated for patients with pretreated advanced NSCLC, regardless of prior taxane exposure or line of therapy.
Keywords: advanced non-small cell lung cancer (NSCLC); multiple treatments; nanoparticle albumin bound paclitaxel (Nab-PTX); secreted protein acidic and rich in cysteine (SPARC); taxane.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures


Similar articles
-
Efficacy and safety of weekly intravenous nanoparticle albumin-bound paclitaxel for non-small cell lung cancer patients who have failed at least two prior systemic treatments.Thorac Cancer. 2017 May;8(3):138-146. doi: 10.1111/1759-7714.12413. Epub 2017 Mar 17. Thorac Cancer. 2017. PMID: 28304139 Free PMC article.
-
Nanoparticle albumin-bound paclitaxel in elder patients with advanced squamous non-small-cell lung cancer: A retrospective study.Cancer Med. 2020 Feb;9(4):1365-1373. doi: 10.1002/cam4.2791. Epub 2019 Dec 26. Cancer Med. 2020. PMID: 31876976 Free PMC article.
-
Efficacy and safety of nanoparticle albumin-bound paclitaxel monotherapy as second-line therapy of cytotoxic anticancer drugs in patients with advanced non-small cell lung cancer.Medicine (Baltimore). 2017 Dec;96(51):e9320. doi: 10.1097/MD.0000000000009320. Medicine (Baltimore). 2017. PMID: 29390506 Free PMC article. Clinical Trial.
-
Albumin-Bound Paclitaxel: A Review in Non-Small Cell Lung Cancer.Drugs. 2015 Nov;75(17):2017-24. doi: 10.1007/s40265-015-0484-9. Drugs. 2015. PMID: 26541764 Review.
-
Nab-paclitaxel Monotherapy for Relapsed Small Cell Lung Cancer: Retrospective Analysis and Review.Anticancer Res. 2020 Mar;40(3):1579-1585. doi: 10.21873/anticanres.14105. Anticancer Res. 2020. PMID: 32132060 Review.
Cited by
-
Multi-targeted tyrosine kinase inhibitors as third-line regimen in advanced non-small cell lung cancer: a network meta-analysis.Ann Transl Med. 2019 Sep;7(18):452. doi: 10.21037/atm.2019.08.51. Ann Transl Med. 2019. PMID: 31700888 Free PMC article.
-
Efficacy and safety of anlotinib in combination with immune checkpoint inhibitors or not as advanced non-small cell lung cancer treatment: a systematic review and network meta-analysis.Transl Cancer Res. 2024 May 31;13(5):2451-2463. doi: 10.21037/tcr-23-1483. Epub 2024 Apr 30. Transl Cancer Res. 2024. PMID: 38881944 Free PMC article.
-
Phase I/II study of biweekly nab-paclitaxel in patients with platinum-pretreated non-small cell lung cancer: NJLCG1402.Thorac Cancer. 2021 Nov;12(21):2886-2893. doi: 10.1111/1759-7714.14149. Epub 2021 Sep 14. Thorac Cancer. 2021. PMID: 34523232 Free PMC article. Clinical Trial.
-
Prognostic significance of SPARC expression in non-small cell lung cancer: A meta-analysis and bioinformatics analysis.Oncol Lett. 2022 Sep 27;24(5):412. doi: 10.3892/ol.2022.13532. eCollection 2022 Nov. Oncol Lett. 2022. PMID: 36245823 Free PMC article.
-
Investigating the relationship between secreted protein acidic and rich in cysteine expression level and therapeutic efficacy of nab-paclitaxel: a meta-analysis.Transl Cancer Res. 2021 Feb;10(2):876-885. doi: 10.21037/tcr-20-3045. Transl Cancer Res. 2021. PMID: 35116417 Free PMC article.
References
-
- Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, Spitznagel EL, Piccirillo J. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–44. - PubMed
-
- Shaw AT, Yeap BY, Solomon BJ, Riely GJ, Gainor J, Engelman JA, Shapiro GI, Costa DB, Ou SH, Butaney M, Salgia R, Maki RG, Varella-Garcia M, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–12. - PMC - PubMed
-
- Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G, Jr, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

