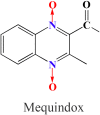
Toxic metabolites, Sertoli cells and Y chromosome related genes are potentially linked to the reproductive toxicity induced by mequindox
- PMID: 29152098
- PMCID: PMC5675650
- DOI: 10.18632/oncotarget.20916
Toxic metabolites, Sertoli cells and Y chromosome related genes are potentially linked to the reproductive toxicity induced by mequindox
Abstract
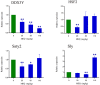
Mequindox (MEQ) is a relatively new synthetic antibacterial agent widely applied in China since the 1980s. However, its reproductive toxicity has not been adequately performed. In the present study, four groups of male Kunming mice (10 mice/group) were fed diets containing MEQ (0, 25, 55 and 110 mg/kg in the diet) for up to 18 months. The results show that M4 could pass through the blood-testis barrier (BTB), and demonstrate that Sertoli cells (SCs) are the main toxic target for MEQ to induce spermatogenesis deficiency. Furthermore, adrenal toxicity, adverse effects on the hypothalamic-pituitary-testicular axis (HPTA) and Leydig cells, as well as the expression of genes related to steroid biosynthesis and cholesterol transport, were responsible for the alterations in sex hormones in the serum of male mice after exposure to MEQ. Additionally, the changed levels of Y chromosome microdeletion related genes, such as DDX3Y, HSF2, Sly and Ssty2 in the testis might be a mechanism for the inhibition of spermatogenesis induced by MEQ. The present study illustrates for the first time the toxic metabolites of MEQ in testis of mice, and suggests that SCs, sex hormones and Y chromosome microdeletion genes are involved in reproductive toxicity mediated by MEQ in vivo.
Keywords: Sertoli cells; Y chromosome microdeletion; blood-testis barrier; mequindox; reproductive toxicity.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that there are no conflicts of interest.
Figures




Similar articles
-
The Reproductive Toxicity of Mequindox in a Two-Generation Study in Wistar Rats.Front Pharmacol. 2018 Aug 17;9:870. doi: 10.3389/fphar.2018.00870. eCollection 2018. Front Pharmacol. 2018. PMID: 30186160 Free PMC article.
-
Mechanisms of the Testis Toxicity Induced by Chronic Exposure to Mequindox.Front Pharmacol. 2017 Sep 26;8:679. doi: 10.3389/fphar.2017.00679. eCollection 2017. Front Pharmacol. 2017. PMID: 29018347 Free PMC article.
-
Fluoride exposure changed the structure and the expressions of Y chromosome related genes in testes of mice.Chemosphere. 2016 Oct;161:292-299. doi: 10.1016/j.chemosphere.2016.06.106. Epub 2016 Jul 18. Chemosphere. 2016. PMID: 27441988
-
Endocrine disruptors and estrogenic effects on male reproductive axis.Asian J Androl. 2008 Jan;10(1):134-45. doi: 10.1111/j.1745-7262.2008.00370.x. Asian J Androl. 2008. PMID: 18087652 Review.
-
Review on testicular development, structure, function, and regulation in common marmoset.Birth Defects Res B Dev Reprod Toxicol. 2005 Oct;74(5):450-69. doi: 10.1002/bdrb.20057. Birth Defects Res B Dev Reprod Toxicol. 2005. PMID: 16193499 Review.
Cited by
-
Mequindox-Induced Kidney Toxicity Is Associated With Oxidative Stress and Apoptosis in the Mouse.Front Pharmacol. 2018 May 1;9:436. doi: 10.3389/fphar.2018.00436. eCollection 2018. Front Pharmacol. 2018. PMID: 29765325 Free PMC article.
-
The Reproductive Toxicity of Mequindox in a Two-Generation Study in Wistar Rats.Front Pharmacol. 2018 Aug 17;9:870. doi: 10.3389/fphar.2018.00870. eCollection 2018. Front Pharmacol. 2018. PMID: 30186160 Free PMC article.
-
Mequindox Induced Genotoxicity and Carcinogenicity in Mice.Front Pharmacol. 2018 Apr 10;9:361. doi: 10.3389/fphar.2018.00361. eCollection 2018. Front Pharmacol. 2018. PMID: 29692735 Free PMC article.
-
A Novel Metabolite as a Hapten to Prepare Monoclonal Antibodies for Rapid Screening of Quinoxaline Drug Residues.Foods. 2022 Oct 21;11(20):3305. doi: 10.3390/foods11203305. Foods. 2022. PMID: 37431055 Free PMC article.
References
-
- Wang X, Martínez MA, Cheng G, Liu Z, Huang L, Dai M, Chen D, Martínez-Larrañaga MR, Anadón A, Yuan Z. The critical role of oxidative stress in the toxicity and metabolism of quinoxaline 1,4-di-N-oxides in vitro and in vivo. Drug Metab Rev. 2016;48:159–82. - PubMed
-
- Ding H, Liu Y, Zeng Z, Si H, Liu K, Liu Y, Yang F, Li Y, Zeng D. Pharmacokinetics of mequindox and one of its major metabolites in chickens after intravenous, intramuscular and oral administration. Res Vet Sci. 2012;93:374–7. - PubMed
-
- Ihsan A, Wang X, Liu Z, Wang Y, Huang X, Liu Y, Yu H, Zhang H, Li T, Yang C, Yuan Z. Long-term mequindox treatment induced endocrine and reproductive toxicity via oxidative stress in male Wistar rats. Toxicol Appl Pharmacol. 2011;252:281–8. - PubMed
-
- Ihsan A, Wang X, Tu HG, Zhang W, Dai MH, Peng DP, Wang YL, Huang LL, Chen DM, Mannan S, Tao YF, Liu ZL, Yuan ZH. Genotoxicity evaluation of Mequindox in different short-term tests. Food Chem Toxicol. 2013;51:330–6. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

