Necdin modulates leukemia-initiating cell quiescence and chemotherapy response
- PMID: 29152105
- PMCID: PMC5675657
- DOI: 10.18632/oncotarget.20999
Necdin modulates leukemia-initiating cell quiescence and chemotherapy response
Abstract
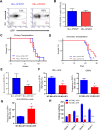
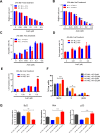
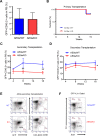
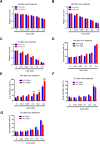
Acute myeloid leukemia (AML) is a devastating illness which carries a very poor prognosis, with most patients living less than 18 months. Leukemia relapse may occur because current therapies eliminate proliferating leukemia cells but fail to eradicate quiescent leukemia-initiating cells (LICs) that can reinitiate the disease after a period of latency. While we demonstrated that p53 target gene Necdin maintains hematopoietic stem cell (HSC) quiescence, its roles in LIC quiescence and response to chemotherapy are unclear. In this study, we utilized two well-established murine models of human AML induced by MLL-AF9 or AML1-ETO9a to determine the role of Necdin in leukemogenesis. We found that loss of Necdin decreased the number of functional LICs and enhanced myeloid differentiation in vivo, leading to delayed development of leukemia induced by MLL-AF9. Importantly, Necdin null LICs expressing MLL-AF9 were less quiescent than wild-type LICs. Further, loss of Necdin enhanced the response of MLL-AF9+ leukemia cells to chemotherapy treatment, manifested by decreased viability and enhanced apoptosis. We observed decreased expression of Bcl2 and increased expression of p53 and its target gene Bax in Necdin null leukemia cells following chemotherapy treatment, indicating that p53-dependent apoptotic pathways may be activated in the absence of Necdin. In addition, we found that loss of Necdin decreased the engraftment of AML1-ETO9a+ hematopoietic stem and progenitor cells in transplantation assays. However, Necdin-deficiency did not affect the response of AML1-ETO9a+ hematopoietic cells to chemotherapy treatment. Thus, Necdin regulates leukemia-initiating cell quiescence and chemotherapy response in a context-dependent manner. Our findings suggest that pharmacological inhibition of Necdin may hold potential as a novel therapy for leukemia patients with MLL translocations.
Keywords: MLL-AF9; Necdin; chemotherapy; leukemia-initiating cells; quiescence.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declared that no conflicts of interest exists.
Figures


Similar articles
-
Necdin, a p53 target gene, regulates the quiescence and response to genotoxic stress of hematopoietic stem/progenitor cells.Blood. 2012 Aug 23;120(8):1601-12. doi: 10.1182/blood-2011-11-393983. Epub 2012 Jul 9. Blood. 2012. PMID: 22776820 Free PMC article.
-
Transient potential receptor melastatin-2 (Trpm2) does not influence murine MLL-AF9-driven AML leukemogenesis or in vitro response to chemotherapy.Exp Hematol. 2016 Jul;44(7):596-602.e3. doi: 10.1016/j.exphem.2016.03.006. Epub 2016 Mar 24. Exp Hematol. 2016. PMID: 27033163 Free PMC article.
-
CD274 promotes cell cycle entry of leukemia-initiating cells through JNK/Cyclin D2 signaling.J Hematol Oncol. 2016 Nov 17;9(1):124. doi: 10.1186/s13045-016-0350-6. J Hematol Oncol. 2016. PMID: 27855694 Free PMC article.
-
Phosphoinositide-dependent kinase 1 regulates leukemia stem cell maintenance in MLL-AF9-induced murine acute myeloid leukemia.Biochem Biophys Res Commun. 2015 Apr 17;459(4):692-8. doi: 10.1016/j.bbrc.2015.03.007. Epub 2015 Mar 11. Biochem Biophys Res Commun. 2015. PMID: 25769952
-
Stem Cell Hierarchy and Clonal Evolution in Acute Lymphoblastic Leukemia.Stem Cells Int. 2015;2015:137164. doi: 10.1155/2015/137164. Epub 2015 Jul 6. Stem Cells Int. 2015. PMID: 26236346 Free PMC article. Review.
Cited by
-
Preclinical Testing in Translational Animal Models of Prader-Willi Syndrome: Overview and Gap Analysis.Mol Ther Methods Clin Dev. 2019 Mar 14;13:344-358. doi: 10.1016/j.omtm.2019.03.001. eCollection 2019 Jun 14. Mol Ther Methods Clin Dev. 2019. PMID: 30989085 Free PMC article. Review.
References
-
- Roboz GJ. Current treatment of acute myeloid leukemia. Curr Opin Oncol. 2012;24:711–19. - PubMed
-
- Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–91. - PubMed
-
- Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–43. - PubMed
-
- Tenen DG. Disruption of differentiation in human cancer: AML shows the way. Nat Rev Cancer. 2003;3:89–101. - PubMed
-
- Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, Kennedy JA, Schimmer AD, Schuh AC, Yee KW, McLeod JL, Doedens M, Medeiros JJ, et al. HALT Pan-Leukemia Gene Panel Consortium Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–33. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

