PSEN1 Mutant iPSC-Derived Model Reveals Severe Astrocyte Pathology in Alzheimer's Disease
- PMID: 29153989
- PMCID: PMC5785689
- DOI: 10.1016/j.stemcr.2017.10.016
PSEN1 Mutant iPSC-Derived Model Reveals Severe Astrocyte Pathology in Alzheimer's Disease
Abstract
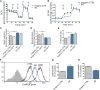
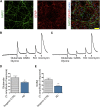
Alzheimer's disease (AD) is a common neurodegenerative disorder and the leading cause of cognitive impairment. Due to insufficient understanding of the disease mechanisms, there are no efficient therapies for AD. Most studies have focused on neuronal cells, but astrocytes have also been suggested to contribute to AD pathology. We describe here the generation of functional astrocytes from induced pluripotent stem cells (iPSCs) derived from AD patients with PSEN1 ΔE9 mutation, as well as healthy and gene-corrected isogenic controls. AD astrocytes manifest hallmarks of disease pathology, including increased β-amyloid production, altered cytokine release, and dysregulated Ca2+ homeostasis. Furthermore, due to altered metabolism, AD astrocytes show increased oxidative stress and reduced lactate secretion, as well as compromised neuronal supportive function, as evidenced by altering Ca2+ transients in healthy neurons. Our results reveal an important role for astrocytes in AD pathology and highlight the strength of iPSC-derived models for brain diseases.
Keywords: calcium homeostasis; cytokine release; lactate secretion; mitochondrial metabolism; oxidative stress; β-amyloid production.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Neurons derived from sporadic Alzheimer's disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation.Alzheimers Res Ther. 2017 Dec 1;9(1):90. doi: 10.1186/s13195-017-0317-z. Alzheimers Res Ther. 2017. PMID: 29191219 Free PMC article.
-
Altered metabolic function induced by Aβ-oligomers and PSEN1 mutations in iPSC-derived astrocytes.J Neurochem. 2025 Jan;169(1):e16267. doi: 10.1111/jnc.16267. J Neurochem. 2025. PMID: 39696767 Free PMC article.
-
Pathological manifestation of the induced pluripotent stem cell-derived cortical neurons from an early-onset Alzheimer's disease patient carrying a presenilin-1 mutation (S170F).Cell Prolif. 2020 Apr;53(4):e12798. doi: 10.1111/cpr.12798. Epub 2020 Mar 25. Cell Prolif. 2020. PMID: 32216003 Free PMC article.
-
Intracellular Calcium Dysregulation: Implications for Alzheimer's Disease.Biomed Res Int. 2016;2016:6701324. doi: 10.1155/2016/6701324. Epub 2016 Jun 2. Biomed Res Int. 2016. PMID: 27340665 Free PMC article. Review.
-
Mitochondrial Calcium Deregulation in the Mechanism of Beta-Amyloid and Tau Pathology.Cells. 2020 Sep 21;9(9):2135. doi: 10.3390/cells9092135. Cells. 2020. PMID: 32967303 Free PMC article. Review.
Cited by
-
Neuromodulation of Glial Function During Neurodegeneration.Front Cell Neurosci. 2020 Aug 21;14:278. doi: 10.3389/fncel.2020.00278. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32973460 Free PMC article.
-
A sporadic Alzheimer's blood-brain barrier model for developing ultrasound-mediated delivery of Aducanumab and anti-Tau antibodies.Theranostics. 2022 Sep 25;12(16):6826-6847. doi: 10.7150/thno.72685. eCollection 2022. Theranostics. 2022. PMID: 36276649 Free PMC article.
-
Prevention and Treatment Strategies for Alzheimer's Disease: Focusing on Microglia and Astrocytes in Neuroinflammation.J Inflamm Res. 2024 Oct 13;17:7235-7259. doi: 10.2147/JIR.S483412. eCollection 2024. J Inflamm Res. 2024. PMID: 39421566 Free PMC article. Review.
-
Modeling Neurological Disorders with Human Pluripotent Stem Cell-Derived Astrocytes.Int J Mol Sci. 2019 Aug 8;20(16):3862. doi: 10.3390/ijms20163862. Int J Mol Sci. 2019. PMID: 31398826 Free PMC article. Review.
-
Human iPSC-Derived Neural Models for Studying Alzheimer's Disease: from Neural Stem Cells to Cerebral Organoids.Stem Cell Rev Rep. 2022 Feb;18(2):792-820. doi: 10.1007/s12015-021-10254-3. Epub 2022 Feb 2. Stem Cell Rev Rep. 2022. PMID: 35107767 Free PMC article. Review.
References
-
- Beal M.F. Oxidative damage as an early marker of Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging. 2005;26:585–586. - PubMed
-
- Belanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. - PubMed
-
- Berridge M.J. Calcium signalling and Alzheimer's disease. Neurochem. Res. 2011;36:1149–1156. - PubMed
-
- Birch A.M. The contribution of astrocytes to Alzheimer's disease. Biochem. Soc. Trans. 2014;42:1316–1320. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

