Dexmedetomidine promotes biomimetic non-rapid eye movement stage 3 sleep in humans: A pilot study
- PMID: 29154132
- PMCID: PMC5743618
- DOI: 10.1016/j.clinph.2017.10.005
Dexmedetomidine promotes biomimetic non-rapid eye movement stage 3 sleep in humans: A pilot study
Abstract
Objectives: Sleep, which comprises of rapid eye movement (REM) and non-REM stages 1-3 (N1-N3), is a natural occurring state of decreased arousal that is crucial for normal cardiovascular, immune and cognitive function. The principal sedative drugs produce electroencephalogram beta oscillations, which have been associated with neurocognitive dysfunction. Pharmacological induction of altered arousal states that neurophysiologically approximate natural sleep, termed biomimetic sleep, may eliminate drug-induced neurocognitive dysfunction.
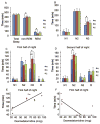
Methods: We performed a prospective, single-site, three-arm, randomized-controlled, crossover polysomnography pilot study (n = 10) comparing natural, intravenous dexmedetomidine- (1-μg/kg over 10 min [n = 7] or 0.5-μg/kg over 10 min [n = 3]), and zolpidem-induced sleep in healthy volunteers. Sleep quality and psychomotor performance were assessed with polysomnography and the psychomotor vigilance test, respectively. Sleep quality questionnaires were also administered.
Results: We found that dexmedetomidine promoted N3 sleep in a dose dependent manner, and did not impair performance on the psychomotor vigilance test. In contrast, zolpidem extended release was associated with decreased theta (∼5-8 Hz; N2 and N3) and increased beta oscillations (∼13-25 Hz; N2 and REM). Zolpidem extended release was also associated with increased lapses on the psychomotor vigilance test. No serious adverse events occurred.
Conclusions: Pharmacological induction of biomimetic N3 sleep with psychomotor sparing benefits is feasible.
Significance: These results suggest that α2a adrenergic agonists may be developed as a new class of sleep enhancing medications with neurocognitive sparing benefits.
Keywords: Biomimetic sleep; Dexmedetomidine; N3 sleep; Sedation; Zolpidem.
Copyright © 2017 International Federation of Clinical Neurophysiology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
O.A. and E.N.B have a provisional patent application describing the use of alpha-2 agonists for promoting N3 sleep. M.T.B has a patent pending on a home sleep monitoring device, has consulting and research agreements with MC10, Insomnisolv, International Flavors and Fragrances, and McKesson Health, he serves as a Medical Monitor for Pfizer, and has provided expert testimony in sleep medicine. E.N.B. has a consulting agreement with Masimo Corporation.
Figures



References
-
- Alexopoulou C, Kondili E, Diamantaki E, Psarologakis C, Kokkini S, Bolaki M, et al. Effects of dexmedetomidine on sleep quality in critically ill patients: a pilot study. Anesthesiology. 2014;121:801–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

