Neurotrophin and FGF Signaling Adapter Proteins, FRS2 and FRS3, Regulate Dentate Granule Cell Maturation and Excitatory Synaptogenesis
- PMID: 29155277
- PMCID: PMC5766409
- DOI: 10.1016/j.neuroscience.2017.11.017
Neurotrophin and FGF Signaling Adapter Proteins, FRS2 and FRS3, Regulate Dentate Granule Cell Maturation and Excitatory Synaptogenesis
Abstract
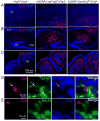
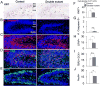
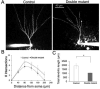
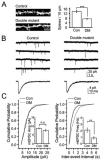
Dentate granule cells (DGCs) play important roles in cognitive processes. Knowledge about how growth factors such as FGFs and neurotrophins contribute to the maturation and synaptogenesis of DGCs is limited. Here, using brain-specific and germline mouse mutants we show that a module of neurotrophin and FGF signaling, the FGF Receptor Substrate (FRS) family of intracellular adapters, FRS2 and FRS3, are together required for postnatal brain development. In the hippocampus, FRS promotes dentate gyrus morphogenesis and DGC maturation during developmental neurogenesis, similar to previously published functions for both neurotrophins and FGFs. Consistent with a role in DGC maturation, two-photon imaging revealed that Frs2,3-double mutants have reduced numbers of dendritic branches and spines in DGCs. Functional analysis further showed that double-mutant mice exhibit fewer excitatory synaptic inputs onto DGCs. These observations reveal roles for FRS adapters in DGC maturation and synaptogenesis and suggest that FRS proteins may act as an important node for FGF and neurotrophin signaling in postnatal hippocampal development.
Keywords: FGF; FRS; hippocampus; neurogenesis; neurotrophin; synaptogenesis.
Copyright © 2017 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Authors declare no conflict of interest.
Figures



Similar articles
-
FGF-Dependent, Context-Driven Role for FRS Adapters in the Early Telencephalon.J Neurosci. 2017 Jun 7;37(23):5690-5698. doi: 10.1523/JNEUROSCI.2931-16.2017. Epub 2017 May 8. J Neurosci. 2017. PMID: 28483978 Free PMC article.
-
Bidirectional Signaling of Neuregulin-2 Mediates Formation of GABAergic Synapses and Maturation of Glutamatergic Synapses in Newborn Granule Cells of Postnatal Hippocampus.J Neurosci. 2015 Dec 16;35(50):16479-93. doi: 10.1523/JNEUROSCI.1585-15.2015. J Neurosci. 2015. PMID: 26674872 Free PMC article.
-
The fibroblast growth factor receptor substrate 3 adapter is a developmentally regulated microtubule-associated protein expressed in migrating and differentiated neurons.J Neurochem. 2010 Feb;112(4):924-39. doi: 10.1111/j.1471-4159.2009.06503.x. Epub 2009 Nov 24. J Neurochem. 2010. PMID: 19943849
-
Adult neurogenesis in the mammalian dentate gyrus.Anat Histol Embryol. 2020 Jan;49(1):3-16. doi: 10.1111/ahe.12496. Epub 2019 Sep 30. Anat Histol Embryol. 2020. PMID: 31568602 Review.
-
Maturation and Functional Integration of New Granule Cells into the Adult Hippocampus.Cold Spring Harb Perspect Biol. 2015 Dec 4;8(1):a018903. doi: 10.1101/cshperspect.a018903. Cold Spring Harb Perspect Biol. 2015. PMID: 26637288 Free PMC article. Review.
Cited by
-
The membrane-linked adaptor FRS2β fashions a cytokine-rich inflammatory microenvironment that promotes breast cancer carcinogenesis.Proc Natl Acad Sci U S A. 2021 Oct 26;118(43):e2103658118. doi: 10.1073/pnas.2103658118. Proc Natl Acad Sci U S A. 2021. PMID: 34663724 Free PMC article.
-
The Interaction of EphA4 With PDGFRβ Regulates Proliferation and Neuronal Differentiation of Neural Progenitor Cells in vitro and Promotes Neurogenesis in vivo.Front Aging Neurosci. 2020 Feb 11;12:7. doi: 10.3389/fnagi.2020.00007. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32116646 Free PMC article.
-
Proteome Analysis of the Hypothalamic Arcuate Nucleus in Chronic High-Fat Diet-Induced Obesity.Biomed Res Int. 2021 Nov 18;2021:3501770. doi: 10.1155/2021/3501770. eCollection 2021. Biomed Res Int. 2021. PMID: 34840970 Free PMC article.
References
-
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. - PubMed
-
- Andersen P, Bliss TV, Skrede KK. Lamellar organization of hippocampal pathways. Exp Brain Res. 1971;13:222–238. - PubMed
-
- Araujo DM, Hilt DC, Miller PJ, Wen D, Jiao S, Lapchak PA. ret receptor tyrosine kinase immunoreactivity is altered in glial cell line-derived neurotrophic factor-responsive neurons following lesions of the nigrostriatal and septohippocampal pathways. Neuroscience. 1997;80:9–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

