Secretion-mediated STAT3 activation promotes self-renewal of glioma stem-like cells during hypoxia
- PMID: 29155422
- PMCID: PMC5851110
- DOI: 10.1038/onc.2017.404
Secretion-mediated STAT3 activation promotes self-renewal of glioma stem-like cells during hypoxia
Abstract
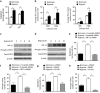
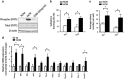
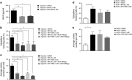
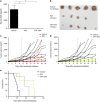
High-grade gliomas (HGGs) include the most common and the most aggressive primary brain tumor of adults and children. Despite multimodality treatment, most high-grade gliomas eventually recur and are ultimately incurable. Several studies suggest that the initiation, progression, and recurrence of gliomas are driven, at least partly, by cancer stem-like cells. A defining characteristic of these cancer stem-like cells is their capacity to self-renew. We have identified a hypoxia-induced pathway that utilizes the Hypoxia Inducible Factor 1α (HIF-1α) transcription factor and the JAK1/2-STAT3 (Janus Kinase 1/2 - Signal Transducer and Activator of Transcription 3) axis to enhance the self-renewal of glioma stem-like cells. Hypoxia is a commonly found pathologic feature of HGGs. Under hypoxic conditions, HIF-1α levels are greatly increased in glioma stem-like cells. Increased HIF-1α activates the JAK1/2-STAT3 axis and enhances tumor stem-like cell self-renewal. Our data further demonstrate the importance of Vascular Endothelial Growth Factor (VEGF) secretion for this pathway of hypoxia-mediated self-renewal. Brefeldin A and EHT-1864, agents that significantly inhibit VEGF secretion, decreased stem cell self-renewal, inhibited tumor growth, and increased the survival of mice allografted with S100β-v-erbB/p53-/- glioma stem-like cells. These agents also inhibit the expression of a hypoxia gene expression signature that is associated with decreased survival of HGG patients. These findings suggest that targeting the secretion of extracellular, autocrine/paracrine mediators of glioma stem-like cell self-renewal could potentially contribute to the treatment of HGGs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994; 367: 645–648. - PubMed
-
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J et al. Identification of a cancer stem cell in human brain tumors. Cancer ResearchCancer ResCancer Res 2003; 63: 5821. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

