A synbiotic-containing amino-acid-based formula improves gut microbiota in non-IgE-mediated allergic infants
- PMID: 29155807
- PMCID: PMC6023699
- DOI: 10.1038/pr.2017.270
A synbiotic-containing amino-acid-based formula improves gut microbiota in non-IgE-mediated allergic infants
Abstract
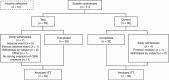
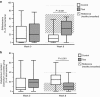
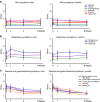
BackgroundPrebiotics and probiotics (synbiotics) can modify gut microbiota and have potential in allergy management when combined with amino-acid-based formula (AAF) for infants with cow's milk allergy (CMA).MethodsThis multicenter, double-blind, randomized controlled trial investigated the effects of an AAF-including synbiotic blend on percentages of bifidobacteria and Eubacterium rectale/Clostridium coccoides group (ER/CC) in feces from infants with suspected non-IgE-mediated CMA. Feces from age-matched healthy breastfed infants were used as reference (healthy breastfed reference (HBR)) for primary outcomes. The CMA subjects were randomized and received test or control formula for 8 weeks. Test formula was a hypoallergenic, nutritionally complete AAF including a prebiotic blend of fructo-oligosaccharides and the probiotic strain Bifidobacterium breve M-16V. Control formula was AAF without synbiotics.ResultsA total of 35 (test) and 36 (control) subjects were randomized; HBR included 51 infants. At week 8, the median percentage of bifidobacteria was higher in the test group than in the control group (35.4% vs. 9.7%, respectively; P<0.001), whereas ER/CC was lower (9.5% vs. 24.2%, respectively; P<0.001). HBR levels of bifidobacteria and ER/CC were 55% and 6.5%, respectively.ConclusionAAF including specific synbiotics, which results in levels of bifidobacteria and ER/CC approximating levels in the HBR group, improves the fecal microbiota of infants with suspected non-IgE-mediated CMA.
Conflict of interest statement
None of the authors have financial ties to products in the study or potential conflict of interest. Authors affiliated to Nutricia Research receive salary, which is not related to study outcome.
Figures
Similar articles
-
Amino Acid Formula Containing Synbiotics in Infants with Cow's Milk Protein Allergy: A Systematic Review and Meta-Analysis.Nutrients. 2021 Mar 14;13(3):935. doi: 10.3390/nu13030935. Nutrients. 2021. PMID: 33799379 Free PMC article.
-
A specific synbiotic-containing amino acid-based formula restores gut microbiota in non-IgE mediated cow's milk allergic infants: a randomized controlled trial.Clin Transl Allergy. 2019 May 31;9:27. doi: 10.1186/s13601-019-0267-6. eCollection 2019. Clin Transl Allergy. 2019. PMID: 31164972 Free PMC article.
-
A specific synbiotic-containing amino acid-based formula in dietary management of cow's milk allergy: a randomized controlled trial.Clin Transl Allergy. 2019 Jan 15;9:5. doi: 10.1186/s13601-019-0241-3. eCollection 2019. Clin Transl Allergy. 2019. PMID: 30651972 Free PMC article.
-
Synbiotics-supplemented amino acid-based formula supports adequate growth in cow's milk allergic infants.Pediatr Allergy Immunol. 2015 Jun;26(4):316-22. doi: 10.1111/pai.12390. Pediatr Allergy Immunol. 2015. PMID: 25845680 Clinical Trial.
-
Prevention of allergic disease in childhood: clinical and epidemiological aspects of primary and secondary allergy prevention.Pediatr Allergy Immunol. 2004 Jun;15 Suppl 16:4-5, 9-32. doi: 10.1111/j.1399-3038.2004.0148b.x. Pediatr Allergy Immunol. 2004. PMID: 15125698 Review.
Cited by
-
Amino Acid Formula Containing Synbiotics in Infants with Cow's Milk Protein Allergy: A Systematic Review and Meta-Analysis.Nutrients. 2021 Mar 14;13(3):935. doi: 10.3390/nu13030935. Nutrients. 2021. PMID: 33799379 Free PMC article.
-
Gut Microbiome Proteomics in Food Allergies.Int J Mol Sci. 2023 Jan 23;24(3):2234. doi: 10.3390/ijms24032234. Int J Mol Sci. 2023. PMID: 36768555 Free PMC article. Review.
-
The Role of Early Life Microbiota Composition in the Development of Allergic Diseases.Microorganisms. 2022 Jun 9;10(6):1190. doi: 10.3390/microorganisms10061190. Microorganisms. 2022. PMID: 35744708 Free PMC article. Review.
-
Insights into Microbiome and Metabolic Signatures of Children Undergoing Peanut Oral Immunotherapy.Children (Basel). 2022 Aug 9;9(8):1192. doi: 10.3390/children9081192. Children (Basel). 2022. PMID: 36010081 Free PMC article.
-
Early antibiotic exposure and development of asthma and allergic rhinitis in childhood.BMC Pediatr. 2019 Jul 5;19(1):225. doi: 10.1186/s12887-019-1594-4. BMC Pediatr. 2019. PMID: 31277618 Free PMC article.
References
-
- Nwaru BI, Hickstein L, Panesar SS et al. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy 2014;69:992–1007. - PubMed
-
- Fiocchi A, Brozek J, Schunemann H et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines. Pediatr Allergy Immunol 2010;21 (Suppl 21): 1–125. - PubMed
-
- Schoemaker AA, Sprikkelman AB, Grimshaw KE et al. Incidence and natural history of challenge-proven cow's milk allergy in European children—EuroPrevall birth cohort. Allergy 2015;70:963–72. - PubMed
-
- Luyt D, Ball H, Makwana N et al. BSACI guideline for the diagnosis and management of cow's milk allergy. Clin Exp Allergy 2014;44:642–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

