Multivariate pattern dependence
- PMID: 29155809
- PMCID: PMC5714382
- DOI: 10.1371/journal.pcbi.1005799
Multivariate pattern dependence
Abstract
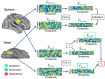
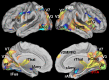
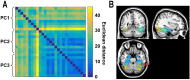
When we perform a cognitive task, multiple brain regions are engaged. Understanding how these regions interact is a fundamental step to uncover the neural bases of behavior. Most research on the interactions between brain regions has focused on the univariate responses in the regions. However, fine grained patterns of response encode important information, as shown by multivariate pattern analysis. In the present article, we introduce and apply multivariate pattern dependence (MVPD): a technique to study the statistical dependence between brain regions in humans in terms of the multivariate relations between their patterns of responses. MVPD characterizes the responses in each brain region as trajectories in region-specific multidimensional spaces, and models the multivariate relationship between these trajectories. We applied MVPD to the posterior superior temporal sulcus (pSTS) and to the fusiform face area (FFA), using a searchlight approach to reveal interactions between these seed regions and the rest of the brain. Across two different experiments, MVPD identified significant statistical dependence not detected by standard functional connectivity. Additionally, MVPD outperformed univariate connectivity in its ability to explain independent variance in the responses of individual voxels. In the end, MVPD uncovered different connectivity profiles associated with different representational subspaces of FFA: the first principal component of FFA shows differential connectivity with occipital and parietal regions implicated in the processing of low-level properties of faces, while the second and third components show differential connectivity with anterior temporal regions implicated in the processing of invariant representations of face identity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Anzellotti Stefano and Caramazza Alfonso From parts to identity: invariance and sensitivity of face representations to different face halves, Cerebral Cortex 2015. 26(5):1900–1909. doi: 10.1093/cercor/bhu337 - DOI - PubMed
-
- Fedorenko Evelina and Thompson-Schill Sharon L Reworking the language network, Trends in cognitive sciences 2014. 18(3):120–126. doi: 10.1016/j.tics.2013.12.006 - DOI - PMC - PubMed
-
- Ishai Alumit Let’s face it: it’s a cortical network, Neuroimage 2008. 40(2):415–419. doi: 10.1016/j.neuroimage.2007.10.040 - DOI - PubMed
-
- Gallagher Helen L and Frith Christopher D Functional imaging of ‘theory of mind’, Trends in cognitive sciences 2003. 7(2):77–83. doi: 10.1016/S1364-6613(02)00025-6 - DOI - PubMed
-
- Le Bihan Denis and Mangin Jean-François and Poupon Cyril and Clark Chris A and Pappata Sabina and Molko Nicolas and Chabriat Hughes Diffusion tensor imaging: concepts and applications, Journal of magnetic resonance imaging 2001. 13(4):534–546. doi: 10.1002/jmri.1076 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

