Dementia After Moderate-Severe Traumatic Brain Injury: Coexistence of Multiple Proteinopathies
- PMID: 29155947
- PMCID: PMC5939622
- DOI: 10.1093/jnen/nlx101
Dementia After Moderate-Severe Traumatic Brain Injury: Coexistence of Multiple Proteinopathies
Abstract
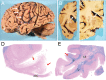
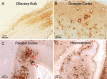
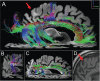
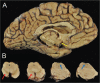
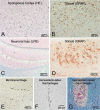
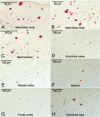
We report the clinical, neuroimaging, and neuropathologic characteristics of 2 patients who developed early onset dementia after a moderate-severe traumatic brain injury (TBI). Neuropathological evaluation revealed abundant β-amyloid neuritic and cored plaques, diffuse β-amyloid plaques, and frequent hyperphosphorylated-tau neurofibrillary tangles (NFT) involving much of the cortex, including insula and mammillary bodies in both cases. Case 1 additionally showed NFTs in both the superficial and deep cortical layers, occasional perivascular and depth-of-sulci NFTs, and parietal white matter rarefaction, which corresponded with decreased parietal fiber tracts observed on ex vivo MRI. Case 2 additionally showed NFT predominance in the superficial layers of the cortex, hypothalamus and brainstem, diffuse Lewy bodies in the cortex, amygdala and brainstem, and intraneuronal TDP-43 inclusions. The neuropathologic diagnoses were atypical Alzheimer disease (AD) with features of chronic traumatic encephalopathy and white matter loss (Case 1), and atypical AD, dementia with Lewy bodies and coexistent TDP-43 pathology (Case 2). These findings support an epidemiological association between TBI and dementia and further characterize the variety of misfolded proteins that may accumulate after TBI. Analyses with comprehensive clinical, imaging, genetic, and neuropathological data are required to characterize the full clinicopathological spectrum associated with dementias occurring after moderate-severe TBI.
Keywords: Hyperphosphorylated tau; Neurodegeneration; Neurofibrillary tangle; Proteinopathy; Traumatic brain injury; α-Synuclein; β-Amyloid.
2017 American Association of Neuropathologists, Inc. This work is written by US Government employees and is in the public domain in the US.
Figures





References
-
- Plassman BL, Havlik RJ, Steffens DC, et al.Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 2000; 55:1158–66 - PubMed
-
- O'Meara ES, Kukull WA, Sheppard L, et al.Head injury and risk of Alzheimer’s disease by apolipoprotein E genotype. Am J Epidemiol 1997; 146:373–84 - PubMed
-
- Mehta KM, Ott A, Kalmijn S, et al.Head trauma and risk of dementia and Alzheimer’s disease: the Rotterdam study. Neurology 1999; 53:1959–62 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

