The Toxmatrix: Chemo-Genomic Profiling Identifies Interactions That Reveal Mechanisms of Toxicity
- PMID: 29156121
- PMCID: PMC9724813
- DOI: 10.1021/acs.chemrestox.7b00290
The Toxmatrix: Chemo-Genomic Profiling Identifies Interactions That Reveal Mechanisms of Toxicity
Abstract
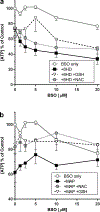
A chemical genomics "Toxmatrix" method was developed to elucidate mechanisms of cytotoxicity using neuronal models. Quantitative high-throughput screening (qHTS) was applied to systematically screen each toxicant against a panel of 70 modulators, drugs or chemicals that act on a known target, to identify interactions that either protect or sensitize cells to each toxicant. Thirty-two toxicants were tested at 10 concentrations for cytotoxicity to SH-SY5Y human neuroblastoma cells, with results fitted to the Hill equation to determine an IC50 for each toxicant. Thirty-three toxicant:modulator interactions were identified in SH-SY5Y cells for 14 toxicants, as modulators that shifted toxicant IC50 values lower or higher. The target of each modulator that sensitizes cells or protects cells from a toxicant suggests a mode of toxicant action or cellular adaptation. In secondary screening, we tested modulator-toxicant pairs identified from the SH-SY5Y primary screening for interactions in three differentiated neuronal human cell lines: dSH-SY5Y, conditionally immortalized dopaminergic neurons (LUHMES), and neural stem cells. Twenty toxicant-modulator pairs showed pronounced interactions in one or several differentiated cell models. Additional testing confirmed that several modulators acted through their primary targets. For example, several chelators protected differentiated LUHMES neurons from four toxicants by chelation of divalent cations and buthionine sulphoximine sensitized cells to 6-hydroxydopamine and 4-(methylamino)phenol hemisulfate by blocking glutathione synthesis. Such modulators that interact with multiple neurotoxicants suggest these may be vulnerable toxicity pathways in neurons. Thus, the Toxmatrix method is a systematic high-throughput approach that can identify mechanisms of toxicity and cellular adaptation.
Figures
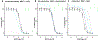



References
-
- Yilmaz C, and Ozcengiz G (2017) Antibiotics: Pharmacokinetics, toxicity, resistance and multidrug efflux pumps. Biochem Pharmacol 133, 43–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

