PP1:Tautomycetin Complex Reveals a Path toward the Development of PP1-Specific Inhibitors
- PMID: 29156132
- PMCID: PMC5729109
- DOI: 10.1021/jacs.7b09368
PP1:Tautomycetin Complex Reveals a Path toward the Development of PP1-Specific Inhibitors
Abstract
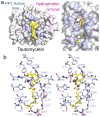
Selective inhibitors for each serine/threonine phosphatase (PPP) are essential to investigate the biological actions of PPPs and to guide drug development. Biologically diverse organisms (e.g., cyanobacteria, dinoflagellates, beetles) produce structurally distinct toxins that are catalytic inhibitors of PPPs. However, most toxins exhibit little selectivity, typically inhibiting multiple family members with similar potencies. Thus, the use of these toxins as chemical tools to study the relationship between individual PPPs and their biological substrates, and how disruptions in these relationships contributes to human disease, is severely limited. Here, we show that tautomycetin (TTN) is highly selective for a single PPP, protein phosphatase 1 (PP1/PPP1C). Our structure of the PP1:TTN complex reveals that PP1 selectivity is defined by a covalent bond between TTN and a PP1-specific cysteine residue, Cys127. Together, these data provide key molecular insights needed for the development of novel probes targeting single PPPs, especially PP1.
Conflict of interest statement
The authors declare no competing financial interest.
Atomic coordinates and structure factors for the PP1:TTN complex have been deposited in the Protein Data Bank (PDB 6ALZ).
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

