The Invasive Region of Glioblastoma Defined by 5ALA Guided Surgery Has an Altered Cancer Stem Cell Marker Profile Compared to Central Tumour
- PMID: 29156557
- PMCID: PMC5713419
- DOI: 10.3390/ijms18112452
The Invasive Region of Glioblastoma Defined by 5ALA Guided Surgery Has an Altered Cancer Stem Cell Marker Profile Compared to Central Tumour
Abstract
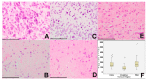
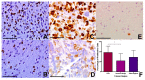
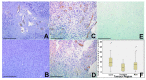
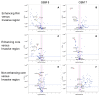
Glioblastoma, a WHO grade IV astrocytoma, is a highly aggressive and heterogeneous tumour that infiltrates deeply into surrounding brain parenchyma, making complete surgical resection impossible. Despite chemo-radiotherapy, the residual cell population within brain parenchyma post-surgery causes inevitable recurrence. Previously, the tumour core has been the focus of research and the basis for targeted therapeutic regimes, which have failed to improve survival in clinical trials. Here, we focus on the invasive margin as defined by the region with 5-aminolevulinic acid (5ALA) (GliolanTM) fluorescence at surgery beyond the T1 enhancing region on magnetic resonance imaging (MRI). This area is hypothesized to constitute unique microenvironmental pressures, and consequently be molecularly distinct to tumour core and enhancing rim regions. We conducted hematoxylin and eosin (H&E), array real time polymerase chain reaction (PCR), and immunohistochemistry staining on various intra-tumour regions of glioblastoma to determine molecular heterogeneity between regions. We analyzed 73 tumour samples from 21 patients and compared cellular density, cell proliferation, and the degree of vascularity. There is a statistically significant difference between the core, invasive margin and other regions for cell density (p < 0.001), cell proliferation (p = 0.029), and vascularity (p = 0.007). Aldehyde dehydrogenase 1 (ALDH1) and Nestin immunohistochemistry were used as a measure of stem-like properties, showing significantly decreased Nestin expression (p < 0.0001) in the invasive margin. Array PCR of the core, rim, and invasive regions showed significantly increased fibroblast growth factor (FGF) and ALDH1 expression in the invasive zone, with elevated hypoxia inducing factor 1-alpha (HIF1α) in the rim region, adjacent to the hypoxic core. The influence of varying microenvironments in the intra-tumour regions is a major key to understanding intra-tumour heterogeneity. This study confirms the distinct molecular composition of the heterogeneous invasive margin and cautions against purported therapy strategies that target candidate glioblastoma stem-like genes that are predominantly expressed in the tumour core. Full characterization of tumour cells in the invasive margin is critical, as these cells may more closely resemble the residual cell population responsible for tumour recurrence. Their unique nature should be considered when developing targeted agents for residual glioblastoma multiforme (GBM).
Keywords: 5ALA; glioma stem cell; hypoxia; intra-tumour heterogeneity; invasion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Stummer W., Pichlmeier U., Meinel T., Wiestler O.D., Zanella F., Reulen H.-J., ALA-Glioma Study Group Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. - DOI - PubMed
-
- Sturm D., Witt H., Hovestadt V., Khuong-Quang D.-A., Jones D.T.W., Konermann C., Pfaff E., Tönjes M., Sill M., Bender S., et al. Hotspot Mutations in H3F3A and IDH1 Define Distinct Epigenetic and Biological Subgroups of Glioblastoma. Cancer Cell. 2012;22:425–437. doi: 10.1016/j.ccr.2012.08.024. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

