Pancreatic Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells
- PMID: 29156578
- PMCID: PMC5744089
- DOI: 10.3390/biomedicines5040065
Pancreatic Cancer: Molecular Characterization, Clonal Evolution and Cancer Stem Cells
Abstract
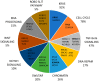
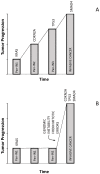
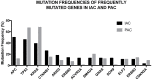
Pancreatic Ductal Adenocarcinoma (PDAC) is the fourth most common cause of cancer-related death and is the most lethal of common malignancies with a five-year survival rate of <10%. PDAC arises from different types of non-invasive precursor lesions: intraductal papillary mucinous neoplasms, mucinous cystic neoplasms and pancreatic intraepithelial neoplasia. The genetic landscape of PDAC is characterized by the presence of four frequently-mutated genes: KRAS, CDKN2A, TP53 and SMAD4. The development of mouse models of PDAC has greatly contributed to the understanding of the molecular and cellular mechanisms through which driver genes contribute to pancreatic cancer development. Particularly, oncogenic KRAS-driven genetically-engineered mouse models that phenotypically and genetically recapitulate human pancreatic cancer have clarified the mechanisms through which various mutated genes act in neoplasia induction and progression and have led to identifying the possible cellular origin of these neoplasias. Patient-derived xenografts are increasingly used for preclinical studies and for the development of personalized medicine strategies. The studies of the purification and characterization of pancreatic cancer stem cells have suggested that a minority cell population is responsible for initiation and maintenance of pancreatic adenocarcinomas. The study of these cells could contribute to the identification and clinical development of more efficacious drug treatments.
Keywords: cancer stem cells; genomic profiling; membrane cell markers; pancreatic ductal adenocarcinoma; tumor xenotransplantation assay.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

