In-Depth Glyco-Peptidomics Approach Reveals Unexpected Diversity of Glycosylated Peptides and Atypical Post-Translational Modifications in Dendroaspis angusticeps Snake Venom
- PMID: 29156586
- PMCID: PMC5713420
- DOI: 10.3390/ijms18112453
In-Depth Glyco-Peptidomics Approach Reveals Unexpected Diversity of Glycosylated Peptides and Atypical Post-Translational Modifications in Dendroaspis angusticeps Snake Venom
Abstract
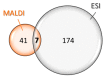
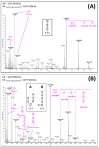
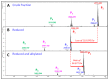
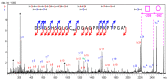
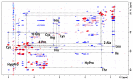
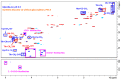
Animal venoms represent a valuable source of bioactive peptides that can be derived into useful pharmacological tools, or even innovative drugs. In this way, the venom of Dendroaspis angusticeps (DA), the Eastern Green Mamba, has been intensively studied during recent years. It mainly contains hundreds of large toxins from 6 to 9 kDa, each displaying several disulfide bridges. These toxins are the main target of venom-based studies due to their valuable activities obtained by selectively targeting membrane receptors, such as ion channels or G-protein coupled receptors. This study aims to demonstrate that the knowledge of venom composition is still limited and that animal venoms contain unexpected diversity and surprises. A previous study has shown that Dendroaspis angusticeps venom contains not only a cocktail of classical toxins, but also small glycosylated peptides. Following this work, a deep exploration of DA glycopeptidome by a dual nano liquid chromatography coupled to electrospray ionization mass spectrometry (nanoLC-ESI-MS) and Matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) analyses was initiated. This study reveals unsuspected structural diversity of compounds such as 221 glycopeptides, displaying different glycan structures. Sequence alignments underline structural similarities with natriuretic peptides already characterized in Elapidae venoms. Finally, the presence of an S-cysteinylation and hydroxylation of proline on four glycopeptides, never described to date in snake venoms, is also revealed by proteomics and affined by nuclear magnetic resonance (NMR) experiments.
Keywords: Dendroaspis angusticeps; glycopeptidome; glycotoxins; venomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Quinton L., Girard E., Maiga A., Rekik M., Lluel P., Masuyer G., Larregola M., Marquer C., Ciolek J., Magnin T., et al. Isolation and pharmacological characterization of AdTx1, a natural peptide displaying specific insurmountable antagonism of the a 1A-adrenoceptor. Br. J. Pharmacol. 2010;159:316–325. doi: 10.1111/j.1476-5381.2009.00532.x. - DOI - PMC - PubMed
-
- Adem A., Asblom A., Johansson G., Mbugua P.M., Karlsson E. Toxins from the venom of the green mamba Dendroaspis angusticeps that inhibit the binding of quinuclidinyl benzilate to muscarinic acetylcholine receptors. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1988;968:340–345. doi: 10.1016/0167-4889(88)90025-0. - DOI - PubMed
-
- Rouget C., Quinton L., Maïga A., Gales C., Masuyer G., Malosse C., Chamot-Rooke J., Thai R., Mourier G., De Pauw E., et al. Identification of a novel snake peptide toxin displaying high affinity and antagonist behaviour for the α2-adrenoceptors. Br. J. Pharmacol. 2010;161:1361–1374. doi: 10.1111/j.1476-5381.2010.00966.x. - DOI - PMC - PubMed
-
- Ciolek J., Reinfrank H., Quinton L., Viengchareun S., Stura E.A., Vera L., Sigismeau S., Mouillac B., Orcel H., Peigneur S., et al. Green mamba peptide targets type-2 vasopressin receptor against polycystic kidney disease. Proc. Natl. Acad. Sci. USA. 2017;114:7154–7159. doi: 10.1073/pnas.1620454114. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

