Akt1 Stimulates Homologous Recombination Repair of DNA Double-Strand Breaks in a Rad51-Dependent Manner
- PMID: 29156644
- PMCID: PMC5713439
- DOI: 10.3390/ijms18112473
Akt1 Stimulates Homologous Recombination Repair of DNA Double-Strand Breaks in a Rad51-Dependent Manner
Abstract
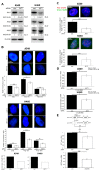
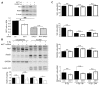
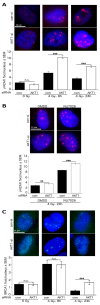
Akt1 is known to promote non-homologous end-joining (NHEJ)-mediated DNA double-strand break (DSB) repair by stimulation of DNA-PKcs. In the present study, we investigated the effect of Akt1 on homologous recombination (HR)-dependent repair of radiation-induced DSBs in non-small cell lung cancer (NSCLC) cells A549 and H460. Akt1-knockdown (Akt1-KD) significantly reduced Rad51 protein level, Rad51 foci formation and its colocalization with γH2AX foci after irradiation. Moreover, Akt1-KD decreased clonogenicity after treatment with Mitomycin C and HR repair, as tested by an HR-reporter assay. Double knockdown of Akt1 and Rad51 did not lead to a further decrease in HR compared to the single knockdown of Rad51. Consequently, Akt1-KD significantly increased the number of residual DSBs after irradiation partially independent of the kinase activity of DNA-PKcs. Likewise, the number of residual BRCA1 foci, indicating unsuccessful HR events, also significantly increased in the irradiated cells after Akt1-KD. Together, the results of the study indicate that Akt1 seems to be a regulatory component in the HR repair of DSBs in a Rad51-dependent manner. Thus, based on this novel role of Akt1 in HR and the previously described role of Akt1 in NHEJ, we propose that targeting Akt1 could be an effective approach to selectively improve the killing of tumor cells by DSB-inducing cytotoxic agents, such as ionizing radiation.
Keywords: Akt1; DNA double-strand break repair; Rad51; homologous recombination; non-small cell lung cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Curran W.J., Jr., Paulus R., Langer C.J., Komaki R., Lee J.S., Hauser S., Movsas B., Wasserman T., Rosenthal S.A., Gore E., et al. Sequential vs. Concurrent chemoradiation for stage iii non-small cell lung cancer: Randomized phase iii trial rtog 9410. J. Natl. Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

