Multiple regulatory aspects of histone methyltransferase EZH2 in Pb-induced neurotoxicity
- PMID: 29156711
- PMCID: PMC5689601
- DOI: 10.18632/oncotarget.19615
Multiple regulatory aspects of histone methyltransferase EZH2 in Pb-induced neurotoxicity
Abstract
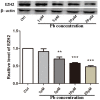
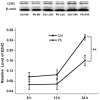
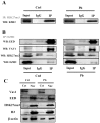
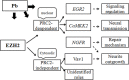
Pb is a pervasive environmental threat to human health. Although remarkable progress has been made in its neurotoxicity, the precise molecular mechanisms underlying this widespread toxicant still remain elusive. In this study, the detailed roles of EZH2, a transcriptional repressor, in the regulation of Pb-led neurotoxicity were investigated, highlighting its sub-functionalization, compartmentalization, functional chaperones and downstream partners. Based on the findings, EZH2's protein levels were significantly reduced in response to Pb treatment; EZH2's gain-of-function trials recovered the dampened neurite outgrowth; EZH2' recruitment to ploycomb complex, as well as its interaction with cytosolic Vav1, was altered in a distinct manner, suggesting that EZH2's multiple roles were markedly redistributed in this context; EZH2's cytosolic and nuclear presence differed in their respective response towards Pb treatment; EZH2 directly occupied the promoters of EGR2, NGFR and CaMKK2, genes responsible for various nerve functions and repair mechanisms, and essentially contributed to their aberrant expression. It indicated that EZH2 mediated the dynamic changes of a cascade of key molecules and consequently the related neurological impairments. In summary, EZH2 emerges as a central player to regulate Pb-led neurotoxicity in a transcriptionally dependent and independent manner, and thereby provided a promising molecular target for medical intervention.
Keywords: EZH2; PRC2; Pb; chromatin immunoprecipitation; neurotoxicity.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures







Similar articles
-
Regulatory Roles of Histone Deacetylases 1 and 2 in Pb-induced Neurotoxicity.Toxicol Sci. 2018 Apr 1;162(2):688-701. doi: 10.1093/toxsci/kfx294. Toxicol Sci. 2018. PMID: 29301062
-
Downregulation of Ezh2 methyltransferase by FOXP3: new insight of FOXP3 into chromatin remodeling?Biochim Biophys Acta. 2013 Oct;1833(10):2190-200. doi: 10.1016/j.bbamcr.2013.05.014. Epub 2013 May 17. Biochim Biophys Acta. 2013. PMID: 23688634
-
FOXA1 antagonizes EZH2-mediated CDKN2A repression in carcinogenesis.Biochem Biophys Res Commun. 2014 Oct 10;453(1):172-8. doi: 10.1016/j.bbrc.2014.09.092. Epub 2014 Sep 27. Biochem Biophys Res Commun. 2014. PMID: 25264199
-
Multifaceted role of the polycomb-group gene EZH2 in hematological malignancies.Int J Hematol. 2017 Jan;105(1):23-30. doi: 10.1007/s12185-016-2124-x. Epub 2016 Nov 9. Int J Hematol. 2017. PMID: 27830540 Review.
-
Roles of the EZH2 histone methyltransferase in cancer epigenetics.Mutat Res. 2008 Dec 1;647(1-2):21-9. doi: 10.1016/j.mrfmmm.2008.07.010. Epub 2008 Aug 3. Mutat Res. 2008. PMID: 18723033 Review.
Cited by
-
Epigenetics: A link between toxicants and diseases.iScience. 2025 May 8;28(6):112613. doi: 10.1016/j.isci.2025.112613. eCollection 2025 Jun 20. iScience. 2025. PMID: 40487436 Free PMC article. Review.
-
Long-term probiotic intervention mitigates memory dysfunction through a novel H3K27me3-based mechanism in lead-exposed rats.Transl Psychiatry. 2020 Jan 22;10(1):25. doi: 10.1038/s41398-020-0719-8. Transl Psychiatry. 2020. PMID: 32066679 Free PMC article.
References
-
- Neal AP, Guilarte TR. Molecular neurobiology of lead ((2+)): effects on synaptic function. Mol Neurobiol. 2010;42:151–60. https://doi.org/10.1007/s12035-010-8146-0. - DOI - PMC - PubMed
-
- Amos-Kroohs RM, Graham DL, Grace CE, Braun AA, Schaefer TL, Skelton MR, Vorhees CV, Williams MT. Developmental stress and lead (Pb): effects of maternal separation and/or Pb on corticosterone, monoamines, and blood Pb in rats. Neurotoxicology. 2016;54:22–33. https://doi.org/10.1016/j.neuro.2016.02.011. - DOI - PMC - PubMed
-
- Senut MC, Cingolani P, Sen A, Kruger A, Shaik A, Hirsch H, Suhr ST, Ruden D. Epigenetics of early-life lead exposure and effects on brain development. Epigenomics. 2012;4:665–74. https://doi.org/10.2217/epi.12.58. - DOI - PMC - PubMed
-
- White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A, Lasley SM, Qian YC, Basha MR. New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol. 2007;225:1–27. https://doi.org/10.1016/j.taap.2007.08.001. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

