A RasGAP, DAB2IP, regulates lipid droplet homeostasis by serving as GAP toward RAB40C
- PMID: 29156729
- PMCID: PMC5689619
- DOI: 10.18632/oncotarget.19960
A RasGAP, DAB2IP, regulates lipid droplet homeostasis by serving as GAP toward RAB40C
Erratum in
-
Correction: A RasGAP, DAB2IP, regulates lipid droplet homeostasis by serving as GAP toward RAB40C.Oncotarget. 2018 Mar 2;9(17):14035. doi: 10.18632/oncotarget.24600. eCollection 2018 Mar 2. Oncotarget. 2018. PMID: 29569654 Free PMC article.
Abstract
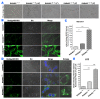
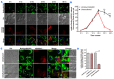
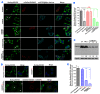
Lipid droplet (LD) homeostasis involves activities of various RAB small GTPases. Recently, we found RAB40C was one of the RAB proteins regulating LD homeostasis. RAB40C contains a unique SOCS domain that is required for clustering of LDs. However, its precise functional role in LD homeostasis and mechanism of regulation remain largely unknown. In this study, we observed over-accumulation of LDs in cells with RAB40C deleted by Crispr-Cas9 editing. RAB40C appeared to reduce LD accumulation after long term incubation of cells with oleic acid (24 hours). Unexpectedly, we found that Ras GTPase activating protein (GAP), DAB2IP, bound to RAB40C mainly via its GAP domain and could serve as RAB40C GAP. Studies involving overexpression of DAB2IP and its GAP defective mutant and siRNA depletion of DAB2IP all confirmed that DAB2IP negatively regulated the effect of RAB40C on LD homeostasis. These results provide a novel perspective on the regulation of RAB40C and implicate various signalling pathways regulated by DAB2IP, which may play a role in LD homeostasis via RAB40C.
Keywords: DAB2IP; GTPase activating protein; RAB40C; lipid droplets.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no competing financial interest.
Figures



References
-
- Wang B, Li L, Fu J, Yu P, Gong D, Zeng C, Zeng Z. Effects of long-chain and medium-chain fatty acids on apoptosis and oxidative stress in human liver cells with steatosis. J Food Sci. 2016;81:H794–800. https://doi.org/10.1111/1750-3841.13210. - DOI - PubMed
-
- Khan SA, Wollaston-Hayden EE, Markowski TW, Higgins L, Mashek DG. Quantitative analysis of the murine lipid droplet-associated proteome during diet-induced hepatic steatosis. J Lipid Res. 2015;56:2260–72. https://doi.org/10.1194/jlr.M056812. - DOI - PMC - PubMed
-
- Wang W, Wei S, Li L, Su X, Du C, Li F, Geng B, Liu P, Xu G. Proteomic analysis of murine testes lipid droplets. Sci Rep. 2015;5:12070. https://doi.org/10.1038/srep12070. - DOI - PMC - PubMed
-
- O’Mahony F, Wroblewski K, O’Byrne SM, Jiang H, Clerkin K, Benhammou J, Blaner WS, Beaven SW. Liver X receptors balance lipid stores in hepatic stellate cells through Rab18, a retinoid responsive lipid droplet protein. Hepatology. 2015;62:615–26. https://doi.org/10.1002/hep.27645. - DOI - PMC - PubMed
-
- Tang WC, Lin RJ, Liao CL, Lin YL. Rab18 facilitates dengue virus infection by targeting fatty acid synthase to sites of viral replication. J Virol. 2014;88:6793–804. https://doi.org/10.1128/jvi.00045-14. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

