Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota
- PMID: 29156761
- PMCID: PMC5689651
- DOI: 10.18632/oncotarget.20689
Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota
Abstract
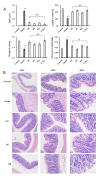
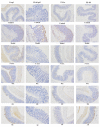
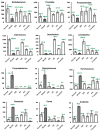
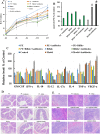
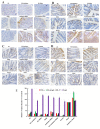
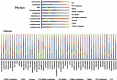
Hericium erinaceus (HE), a traditional edible mushroom, is known as a medicine food homology to ameliorate gastrointestinal diseases. To investigate whether HE is clinically effective in alleviating inflammatory bowel disease (IBD), HE extracts (polysaccharide, alcoholic extracts and whole extracts were prepared using solvent extraction methods) were administrated for 2 weeks in rats with IBD induced by trinitro-benzene-sulfonic acid (TNBS) enema (150 mg/kg). Significant clinical and histological changes in IBD rats were identified, including damage activity, common morphous and tissue damage index scores in colonic mucosa and myeloperoxidase (MPO) activity. The damage activity, common morphous and tissue damage index scores in colonic mucosa (P <0.05) were improved, MPO activities were decreased. Inflammatory factors were also differentially expressed in colonic mucosa in IBD rats, including serum cytokines, Foxp3 and interleukin (IL)-10 were increased while NF-κB p65 and tumor necrosis factor (TNF)-α were decreased (P <0.05), and T cells were activated (P <0.05), especially in the alcohol extracts-treated group. We also found that the structure of gut microbiota of the H. erinaceus extracts-treated groups changed significantly by compared with the model group. Further studies revealed that the polysaccharides in HE extracts may play a prebiotic role, whereas the alcoholic extracts show bactericidin-like and immunomodulatory effects. Taken together, we demonstrated that H. erinaceus extracts could promote the growth of beneficial gut bacteria and improve the host immunity in vivo IBD model, which shows clinical potential in relieving IBD by regulating gut microbiota and immune system.
Keywords: Hericium erinaceus; anti-inflammatory; gut microbiota; immune-enhancing effect; inflammatory bowel disease.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures




Similar articles
-
Immunomodulatory Activities of a Fungal Protein Extracted from Hericium erinaceus through Regulating the Gut Microbiota.Front Immunol. 2017 Jun 12;8:666. doi: 10.3389/fimmu.2017.00666. eCollection 2017. Front Immunol. 2017. PMID: 28713364 Free PMC article.
-
A unique polysaccharide from Hericium erinaceus mycelium ameliorates acetic acid-induced ulcerative colitis rats by modulating the composition of the gut microbiota, short chain fatty acids levels and GPR41/43 respectors.Int Immunopharmacol. 2019 Jun;71:411-422. doi: 10.1016/j.intimp.2019.02.038. Epub 2019 May 3. Int Immunopharmacol. 2019. PMID: 31059977
-
Influence of Short-Term Consumption of Hericium erinaceus on Serum Biochemical Markers and the Changes of the Gut Microbiota: A Pilot Study.Nutrients. 2021 Mar 21;13(3):1008. doi: 10.3390/nu13031008. Nutrients. 2021. PMID: 33800983 Free PMC article.
-
NF-kappaB in inflammatory bowel disease.J Intern Med. 2008 Jun;263(6):591-6. doi: 10.1111/j.1365-2796.2008.01953.x. J Intern Med. 2008. PMID: 18479258 Review.
-
Recent progress on the role of gut microbiota in the pathogenesis of inflammatory bowel disease.J Dig Dis. 2013 Oct;14(10):513-7. doi: 10.1111/1751-2980.12087. J Dig Dis. 2013. PMID: 23848393 Review.
Cited by
-
Metabolites of medicine food homology-derived endophytic fungi and their activities.Curr Res Food Sci. 2022 Oct 7;5:1882-1896. doi: 10.1016/j.crfs.2022.10.006. eCollection 2022. Curr Res Food Sci. 2022. PMID: 36276242 Free PMC article. Review.
-
Immunomodulatory Effect of Cordyceps militaris Polysaccharide on RAW 264.7 Macrophages by Regulating MAPK Signaling Pathways.Molecules. 2024 Jul 20;29(14):3408. doi: 10.3390/molecules29143408. Molecules. 2024. PMID: 39064986 Free PMC article.
-
Association between intestinal microbiome and inflammatory bowel disease: Insights from bibliometric analysis.Comput Struct Biotechnol J. 2022 Apr 7;20:1716-1725. doi: 10.1016/j.csbj.2022.04.006. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35495114 Free PMC article.
-
Antitumor, Anti-Inflammatory and Antiallergic Effects of Agaricus blazei Mushroom Extract and the Related Medicinal Basidiomycetes Mushrooms, Hericium erinaceus and Grifola frondosa: A Review of Preclinical and Clinical Studies.Nutrients. 2020 May 8;12(5):1339. doi: 10.3390/nu12051339. Nutrients. 2020. PMID: 32397163 Free PMC article. Review.
-
Agrocybe cylindracea Polysaccharides Ameliorate DSS-Induced Colitis by Restoring Intestinal Barrier Function and Reprogramming Immune Homeostasis via the Gut-Liver Axis.Int J Mol Sci. 2025 Jul 16;26(14):6805. doi: 10.3390/ijms26146805. Int J Mol Sci. 2025. PMID: 40725052 Free PMC article.
References
-
- Singh D, Srivastava S, Pradhan M, Kanwar JR, Singh MR. Inflammatory bowel disease: pathogenesis, causative factors, issues, drug treatment strategies, and delivery approaches. Crit Rev Ther Drug Carrier Syst. 2015;32:181–214. - PubMed
-
- Burisch J, Munkholm P. The epidemiology of inflammatory bowel disease. Scand J Gastroenterol. 2015;50:942–51. - PubMed
-
- Burisch J. Crohn's disease and ulcerative colitis. Occurrence, course and prognosis during the first year of disease in a European population-based inception cohort. Dan Med J. 2014;61:B4778. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

