Monophosphoryl lipid a attenuates radiation injury through TLR4 activation
- PMID: 29156775
- PMCID: PMC5689665
- DOI: 10.18632/oncotarget.20907
Monophosphoryl lipid a attenuates radiation injury through TLR4 activation
Abstract
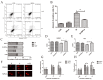
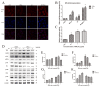
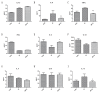
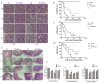
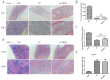
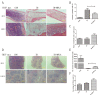
Ionizing radiation causes severe damage to human body, and normal tissue toxicity in cancer radiotherapy also limits its further application. It is urgently required to develop safe and effective radioprotector. Our previous study has shown that toll like receptor 4 (TLR4) was dispensable for basal radiation resistance. However, severe toxicity of its traditional agonist lipopolysaccharide limits the clinical application. In present study, we demonstrated that monophosphoryl lipid A (MPLA), a potent TLR4 agonist with low toxicity, effectively attenuated radiation injury on in vitro and in vivo. MPLA increased cell survival and inhibited cell apoptosis after irradiation, and cell cycle arrest was also inhibited. Radiosensitive tissues including spleen, intestine, bone marrow and testis were protected from radiation damages in a TLR4 dependent manner. We also found that myeloid differentiation factor 88 (MyD88) accounted more than Toll/IL-1R domain-containing adaptor inducing IFN-β (TRIF) for the radioprotective effects of MPLA. In conclusion, our finding suggests TLR4 agonist MPLA as a safe and effective radioprotector for clinical application.
Keywords: MyD88; TRIF; monophosphoryl lipid a (MPLA); radioprotection; toll like receptor 4 (TLR4).
Conflict of interest statement
CONFLICTS OF INTEREST The authors have no conflicts of interest to disclose.
Figures



Similar articles
-
Monophosphoryl lipid A alleviated radiation-induced testicular injury through TLR4-dependent exosomes.J Cell Mol Med. 2020 Apr;24(7):3917-3930. doi: 10.1111/jcmm.14978. Epub 2020 Mar 5. J Cell Mol Med. 2020. PMID: 32135028 Free PMC article.
-
Heat-killed salmonella typhimurium (HKST) protects mice against radiation in TLR4-dependent manner.Oncotarget. 2017 May 15;8(40):67082-67093. doi: 10.18632/oncotarget.17859. eCollection 2017 Sep 15. Oncotarget. 2017. PMID: 28978017 Free PMC article.
-
Endothelial cell tolerance to lipopolysaccharide challenge is induced by monophosphoryl lipid A.Clin Sci (Lond). 2016 Mar;130(6):451-61. doi: 10.1042/CS20150592. Epub 2015 Dec 15. Clin Sci (Lond). 2016. PMID: 26669797 Free PMC article.
-
The role of MyD88- and TRIF-dependent signaling in monophosphoryl lipid A-induced expansion and recruitment of innate immunocytes.J Leukoc Biol. 2016 Dec;100(6):1311-1322. doi: 10.1189/jlb.1A0216-072R. Epub 2016 Jun 27. J Leukoc Biol. 2016. PMID: 27354411 Free PMC article.
-
The Troll in Toll: Mal and Tram as bridges for TLR2 and TLR4 signaling.J Leukoc Biol. 2007 Aug;82(2):196-203. doi: 10.1189/jlb.1206750. Epub 2007 Apr 20. J Leukoc Biol. 2007. PMID: 17449723 Review.
Cited by
-
Emerging clinical relevance of microbiome in cancer: promising biomarkers and therapeutic targets.Protein Cell. 2024 Apr 1;15(4):239-260. doi: 10.1093/procel/pwad052. Protein Cell. 2024. PMID: 37946397 Free PMC article. Review.
-
Monophosphoryl lipid A alleviated radiation-induced testicular injury through TLR4-dependent exosomes.J Cell Mol Med. 2020 Apr;24(7):3917-3930. doi: 10.1111/jcmm.14978. Epub 2020 Mar 5. J Cell Mol Med. 2020. PMID: 32135028 Free PMC article.
-
TLR4 Agonist Monophosphoryl Lipid A Alleviated Radiation-Induced Intestinal Injury.J Immunol Res. 2019 Jun 3;2019:2121095. doi: 10.1155/2019/2121095. eCollection 2019. J Immunol Res. 2019. PMID: 31275998 Free PMC article.
-
S100A8 and S100A9 Promote Apoptosis of Chronic Eosinophilic Leukemia Cells.Front Immunol. 2020 Aug 6;11:1258. doi: 10.3389/fimmu.2020.01258. eCollection 2020. Front Immunol. 2020. PMID: 32903598 Free PMC article.
-
Targets for protection and mitigation of radiation injury.Cell Mol Life Sci. 2020 Aug;77(16):3129-3159. doi: 10.1007/s00018-020-03479-x. Epub 2020 Feb 18. Cell Mol Life Sci. 2020. PMID: 32072238 Free PMC article. Review.
References
-
- Pellmar TC, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–23. - PubMed
-
- Cormier AC, Drapek L, Fahey J, Rowen B, Burns-Britton B, Lavadinho-Lemos M, Hultman T. When the Patient Seeks Cure: Challenging Chemotherapy and Radiation Side Effects Requiring Creative Solutions. Clin J Oncol Nurs. 2016;20:117–20. - PubMed
-
- Shakhov AN, Singh VK, Bone F, Cheney A, Kononov Y, Krasnov P, Bratanova-Toshkova TK, Shakhova VV, Young J, Weil MM, Panoskaltsis-Mortari A, Orschell CM, Baker PS, et al. Prevention and mitigation of acute radiation syndrome in mice by synthetic lipopeptide agonists of Toll-like receptor 2 (TLR2) PLoS One. 2012;7:e33044. https://doi.org/10.1371/journal.pone.0033044. - DOI - PMC - PubMed
-
- Yan L, Xu G, Qiao T, Chen W, Yuan S, Li X. CpG-ODN 7909 increases radiation sensitivity of radiation-resistant human lung adenocarcinoma cell line by overexpression of Toll-like receptor 9. Cancer Biother Radiopharm. 2013;28:559–64. https://doi.org/10.1089/cbr.2012.1450. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

