Tube feeding decreases pneumonia rate in patients with severe dementia: comparison between pre- and post-intervention
- PMID: 29157223
- PMCID: PMC5697435
- DOI: 10.1186/s12877-017-0662-6
Tube feeding decreases pneumonia rate in patients with severe dementia: comparison between pre- and post-intervention
Abstract
Background: It is widely supposed that there is no benefit, including extended survival and decreased rate of pneumonia, in patients with severe dementia receiving enteral tube feeding (TF). However, there have been few studies comparing the frequency of pneumonia before and after TF in severe dementia.
Methods: Nine psychiatric hospitals in Okayama Prefecture participated in this retrospective survey. All inpatients fulfilling the entry criteria were evaluated. All subjects suffered from difficulty in oral intake. Attending physicians thought that the patients could not live without long-term artificial nutrition, and they decided whether or not to make use of long-term artificial nutrition from January 1, 2014 to December 31, 2014.
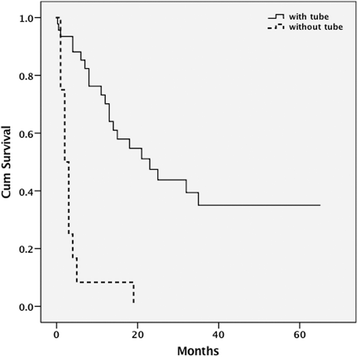
Results: We evaluated 58 patients including 46 with TF and 12 without. The mean age of all patients was 79.6 ± 9.0 years old. Patients with probable Alzheimer's disease (n = 38) formed the biggest group, and those with vascular dementia the second (n = 14). Median survival times were 23 months among patients with TF and two months among patients without TF. The start of TF decreased the frequency of pneumonia and the use of intravenous antibiotics.
Conclusions: TF decreased pneumonia and antibiotic use, even in patients with severe dementia. The results of this study do not necessarily indicate that we should administer TF to patients with severe dementia. We should consider the quality of life of patients carefully before deciding the use or disuse of TF for patients with severe dementia.
Keywords: Dementia; Nasogastric tube; Percutaneous endoscopic gastrostomy; Pneumonia; Tube feeding.
Conflict of interest statement
Ethics approval and consent to participate
This study adhered to the 1975 Helsinki Declaration of Human Rights. The study protocol with a list of participating psychiatric hospitals was approved by the Internal Ethical Committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences (approval number: 1506–005).
All the hospitals taking part in this study agreed to be involved. All the participants completing the survey consented to be part of this study. The representative (geriatric psychiatrist) of this project at each hospital displayed posters on the bulletin board in the participating ward during the study period with an explanation of the project and informing patients and their families that they could decline to participate in this study. Participant consent was not required because data was de-identified prior to analysis.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous