Immunoproteomic analysis of the excretory-secretory products of Trichinella pseudospiralis adult worms and newborn larvae
- PMID: 29157262
- PMCID: PMC5697079
- DOI: 10.1186/s13071-017-2522-9
Immunoproteomic analysis of the excretory-secretory products of Trichinella pseudospiralis adult worms and newborn larvae
Abstract
Background: The nematode Trichinella pseudospiralis is an intracellular parasite of mammalian skeletal muscle cells and exists in a non-encapsulated form. Previous studies demonstrated that T. pseudospiralis could induce a lower host inflammatory response. Excretory-secretory (ES) proteins as the most important products of host-parasite interaction may play the main functional role in alleviating host inflammation. However, the ES products of T. pseudospiralis early stage are still unknown. The identification of the ES products of the early stage facilitates the understanding of the molecular mechanisms of the immunomodulation and may help finding early diagnostic markers.
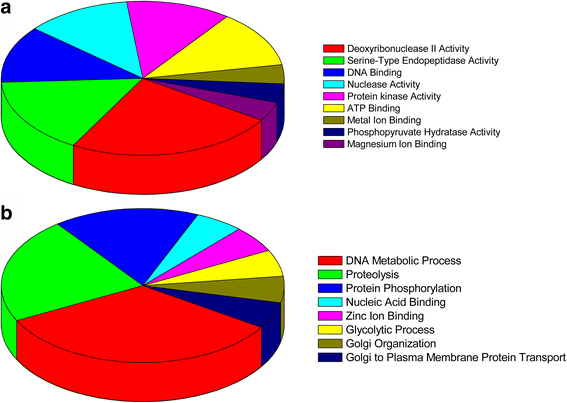
Results: In this study, we used two-dimensional gel electrophoresis (2-DE)-based western blotting coupled with matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF/TOF-MS/MS) to separate and identify the T. pseudospiralis adult worms ES products immunoreaction-positive proteins. In total, 400 protein spots were separated by 2-DE. Twenty-eight protein spots were successfully identified using the sera from infected pigs and were characterized to correlate with 12 different proteins of T. pseudospiralis, including adult-specific DNase II-10, poly-cysteine and histidine-tailed protein isoform 2, serine protease, serine/threonine-protein kinase ULK3, enolase, putative venom allergen 5, chymotrypsin-like elastase family member 1, uncharacterized protein, peptidase inhibitor 16, death-associated protein 1, deoxyribonuclease II superfamily and golgin-45. Bioinformatic analyses showed that the identified proteins have a wide diversity of molecular functions, especially deoxyribonuclease II (DNase II) activity and serine-type endopeptidase activity.
Conclusions: Early candidate antigens from the ES proteins of T. pseudospiralis have been screened and identified. Our results suggest these proteins may play key roles during the T. pseudospiralis infection and suppress the host immune response. Further, they are the most likely antigen for early diagnosis and the development of a vaccine against the parasite.
Keywords: Excretory-secretory proteins; Immunoproteomics; Trichinella pseudospiralis.
Conflict of interest statement
Ethics approval
The study of using laboratory animals was reviewed and approved by the Ethical Committee of Jilin University affiliated to the Provincial Animal Health Committee, Jilin Province, China (Ethical Clearance number IZ-2009-008). All experiments were performed in strict accordance with the requirements of the Animal Ethics Procedures and Guidelines of the People’s Republic of China.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Dupouy-Camet J. Presidential address of ICT12 conference: "Trichinella and trichinellosis - a never ending story". Vet Parasitol. 2009;159(3–4):194–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

