Autologous platelet-rich plasma induces bone formation of tissue-engineered bone with bone marrow mesenchymal stem cells on beta-tricalcium phosphate ceramics
- PMID: 29157270
- PMCID: PMC5697349
- DOI: 10.1186/s13018-017-0665-1
Autologous platelet-rich plasma induces bone formation of tissue-engineered bone with bone marrow mesenchymal stem cells on beta-tricalcium phosphate ceramics
Abstract
Background: The purpose of the study is to investigate whether autologous platelet-rich plasma (PRP) can serve as bone-inducing factors to provide osteoinduction and improve bone regeneration for tissue-engineered bones fabricated with bone marrow mesenchymal stem cells (MSCs) and beta-tricalcium phosphate (β-TCP) ceramics. The current study will give more insight into the contradictory osteogenic capacity of PRP.
Methods: The concentration of platelets, platelet-derived growth factor-AB (PDGF-AB), and transforming growth factor-β1 (TGF-β1) were measured in PRP and whole blood. Tissue-engineered bones using MSCs on β-TCP scaffolds in combination with autologous PRP were fabricated (PRP group). Controls were established without the use of autologous PRP (non-PRP group). In vitro, the proliferation and osteogenic differentiation of MSCs on fabricated constructs from six rabbits were evaluated with MTT assay, alkaline phosphatase (ALP) activity, and osteocalcin (OC) content measurement after 1, 7, and 14 days of culture. For in vivo study, the segmental defects of radial diaphyses of 12 rabbits from each group were repaired by fabricated constructs. Bone-forming capacity of the implanted constructs was determined by radiographic and histological analysis at 4 and 8 weeks postoperatively.
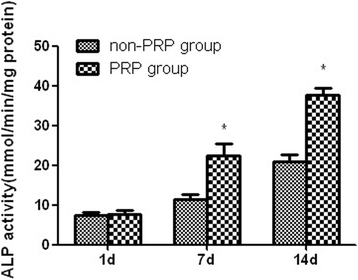
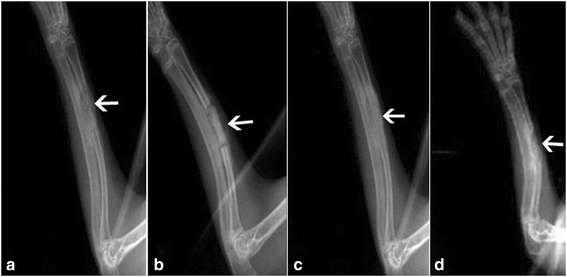
Results: PRP produced significantly higher concentration of platelets, PDGF-AB, and TGF-β1 than whole blood. In vitro study, MTT assay demonstrated that the MSCs in the presence of autologous PRP exhibited excellent proliferation at each time point. The results of osteogenic capacity detection showed significantly higher levels of synthesis of ALP and OC by the MSCs in combination with autologous PRP after 7 and 14 days of culture. In vivo study, radiographic observation showed that the PRP group produced significantly higher score than the non-PRP group at each time point. For histological evaluation, significantly higher volume of regenerated bone was found in the PRP group when compared with the non-PRP group at each time point.
Conclusions: Our study findings support the osteogenic capacity of autologous PRP. The results indicate that the use of autologous PRP is a simple and effective way to provide osteoinduction and improve bone regeneration for tissue-engineered bone reconstruction.
Keywords: Autologous; Beta-tricalcium phosphate; Osteogenic; Platelet-rich plasma; Scaffold; Tissue-engineered bone.
Conflict of interest statement
Ethics approval
The present research was approved by the Qingdao University Medical Ethics Committee. All experimental procedures were in compliance with the principles of International Laboratory Animal Care and with the European Communities Council Directive (86/809 /EEC).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

