Biomarkers for immunotherapy in bladder cancer: a moving target
- PMID: 29157296
- PMCID: PMC5697433
- DOI: 10.1186/s40425-017-0299-1
Biomarkers for immunotherapy in bladder cancer: a moving target
Abstract
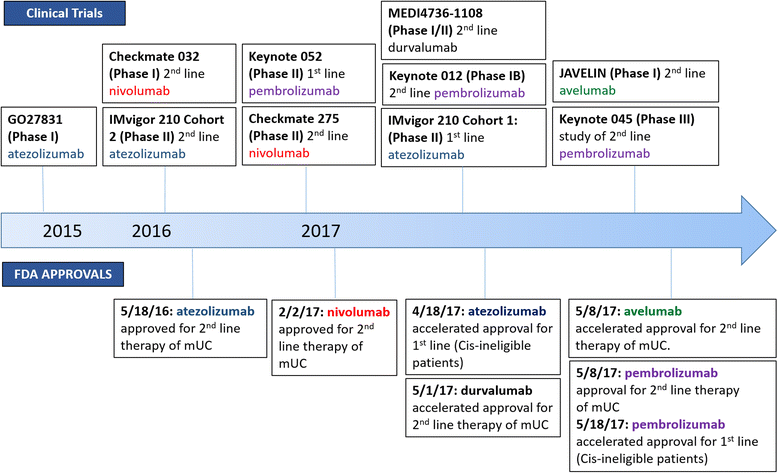
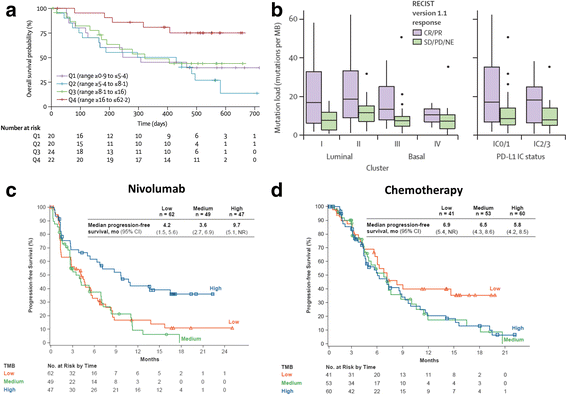
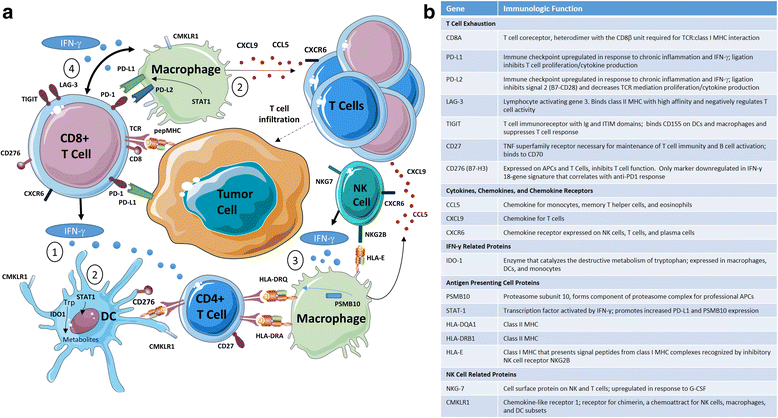
Treatment options for metastatic urothelial carcinoma (mUC) remained relative unchanged over the last 30 years with combination chemotherapy as the mainstay of treatment. Within the last year the landscape for mUC has seismically shifted following the approval of five therapies targeting the programmed cell death protein (PD-1)/programmed cell death ligand 1 (PD-L1) axis. Notably, the anti-PD-1 antibody pembrolizumab demonstrated improved OS relative to chemotherapy in a randomized phase III study for second line treatment of mUC; this level 1 evidence led to approval from the U.S. Food and Drug Administration (FDA). The PD-1 antibody nivolumab also demonstrated an overall survival benefit, in this case in comparison to historical controls. Similarly, antibodies targeting PD-L1 including atezolizumab, durvalumab, and avelumab have now received accelerated approval from the FDA as second line treatments for mUC, with durable response lasting more than 1 year in some patients. Some of these agents are approved in the first line setting as well - based on single-arm phase II studies atezolizumab and pembrolizumab received accelerated approval for first-line treatment of cisplatin ineligible patients. Despite these multiple approvals, the development of clinically useful biomarkers to determine the optimal treatment for patients remains somewhat elusive. In this review, we examine key clinical trial results with anti-PD1/PD-L1 antibodies and discuss progress towards developing novel biomarkers beyond PD-L1 expression.
Keywords: Atezolizumab; Avelumab; Biomarker; Bladder cancer; Durvalumab; Immune checkpoint inhibitor; Immunotherapy; Nanostring; Nivolumab; PD-1; Pd-L1; Pembrolizumab; Tumor mutation burden.
Conflict of interest statement
Authors’ information
DHA is currently a Postdoctoral Clinical Fellow in Hematology and Oncology at Columbia University Medical Center with a research interest in genitourinary oncology and the identification and application of novel immunotherapies. CGD is a Professor of Medicine at Columbia University Medical Center where he serves as the Director of the Genitourinary Oncology Program. He has been senior author and principal investigator on numerous clinical trials in the identification of novel immune checkpoint molecules and the clinical development of multiple immune checkpoint inhibitors.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
DH. Aggen has no competing interests to report. CGD has stock or ownership in Compugen, Potenza Therapeutics, and Tizona Therapeutics. He has served in an advisory role or received research funding from Bristol-Myers Squibb, Compugen, Dendreon, Merck, Genentech, Eli Lilly, NexImmune, ImmuneXcite, Potenza Therapeutics, Tizona Therapeutics, Janssen Oncology (Inst), Regeneron and AstraZeneca/MedImmune.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Surveillance, Epidemiology, and End Results Program . SEER stat fact sheets: kidney and renal pelvis cancer. Bethesda: National Cancer Institute; 2017.
-
- Milowsky MI, Rumble RB, Booth CM, et al. Guideline on muscle-invasive and metastatic bladder cancer (European Association of Urology guideline): American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2016;34:1945–1952. doi: 10.1200/JCO.2015.65.9797. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
