Resident fibroblast expansion during cardiac growth and remodeling
- PMID: 29158033
- PMCID: PMC5831691
- DOI: 10.1016/j.yjmcc.2017.11.012
Resident fibroblast expansion during cardiac growth and remodeling
Abstract
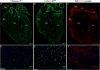
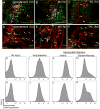
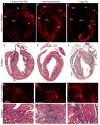
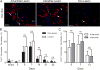
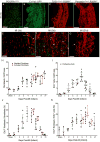
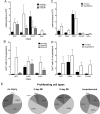
Cardiac fibrosis, denoted by the deposition of extracellular matrix, manifests with a variety of diseases such as hypertension, diabetes, and myocardial infarction. Underlying this pathological extracellular matrix secretion is an expansion of fibroblasts. The mouse is now a common experimental model system for the study of cardiovascular remodeling and elucidation of fibroblast responses to cardiac growth and stress is vital for understanding disease processes. Here, using diverse but fibroblast specific markers, we report murine fibroblast distribution and proliferation in early postnatal, adult, and injured hearts. We find that perinatal fibroblasts and endothelial cells proliferate at similar rates. Furthermore, regardless of the injury model, fibroblast proliferation peaks within the first week after injury, a time window similar to the period of the inflammatory phase. In addition, fibroblast densities remain high weeks after the initial insult. These results provide detailed information regarding fibroblast distribution and proliferation in experimental methods of heart injury.
Keywords: Cardiac fibroblast; Endothelial cell; Fibrosis; Proliferation.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures



References
-
- Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15–26. - PubMed
-
- Kaur H, Takefuji M, Ngai C, Carvalho J, Bayer J, Wietelmann A, et al. Targeted Ablation of Periostin-Expressing Activated Fibroblasts Prevents Adverse Cardiac Remodeling in Mice. Circ Res. 2016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

