Topical mevastatin promotes wound healing by inhibiting the transcription factor c-Myc via the glucocorticoid receptor and the long non-coding RNA Gas5
- PMID: 29158265
- PMCID: PMC5787818
- DOI: 10.1074/jbc.M117.811240
Topical mevastatin promotes wound healing by inhibiting the transcription factor c-Myc via the glucocorticoid receptor and the long non-coding RNA Gas5
Abstract
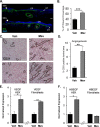
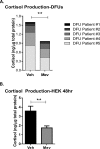
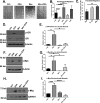
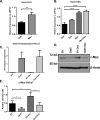
Diabetic foot ulcers (DFUs), a life-threatening complication of diabetes mellitus, have limited treatment options, often resulting in amputations. HMG-CoA reductase inhibitors such as statins are cholesterol-reducing agents that may provide a new therapeutic option. Statins target the cholesterol pathway and block the synthesis of the wound-healing inhibitors farnesyl pyrophosphate (FPP) and cortisol, ligands for the glucocorticoid receptor (GR). Here we demonstrate that the naturally occurring statin mevastatin reverses FPP's effects and promotes healing by using in vitro wound healing assays, human ex vivo and porcine in vivo wound models, and DFU tissue. Moreover, we measured cortisol levels by ELISA and found that mevastatin inhibited cortisol synthesis in keratinocytes and biopsies from patients with DFU. Of note, topical mevastatin stimulated epithelialization and angiogenesis in vivo Mevastatin also reversed FPP-mediated induction of the GR target, the transcription factor c-Myc (a biomarker of non-healing wounds), in porcine and human wound models. Importantly, mevastatin reversed c-Myc overexpression in DFUs. It induced expression of the long noncoding RNA Gas5 that blocks c-Myc expression, which was confirmed by overexpression studies. We conclude that topical mevastatin accelerates wound closure by promoting epithelialization via multiple mechanisms: modulation of GR ligands and induction of the long noncoding RNA Gas5, leading to c-Myc inhibition. In light of these findings, we propose that repurposing statin drugs for topical treatment of DFUs may offer another option for managing this serious condition.
Keywords: Gas5; Myc (c-Myc); diabetic foot ulcers; glucocorticoid receptor; long noncoding RNA (long ncRNA, lncRNA); skin; statin; wound healing.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
M. T. C. is listed as the inventor of Patent PCT/US2010/062361, “Composition and Methods for Promoting Epithelialization and Wound Closure,” issued to New York University based on the data presented, in part, in this study and stands to potentially gain royalties from future commercialization
Figures

Similar articles
-
Farnesyl pyrophosphate inhibits epithelialization and wound healing through the glucocorticoid receptor.J Biol Chem. 2010 Jan 15;285(3):1980-8. doi: 10.1074/jbc.M109.016741. Epub 2009 Nov 10. J Biol Chem. 2010. PMID: 19903814 Free PMC article.
-
Mevastatin promotes healing by targeting caveolin-1 to restore EGFR signaling.JCI Insight. 2019 Dec 5;4(23):e129320. doi: 10.1172/jci.insight.129320. JCI Insight. 2019. PMID: 31661463 Free PMC article.
-
LncRNA GAS5 activates the HIF1A/VEGF pathway by binding to TAF15 to promote wound healing in diabetic foot ulcers.Lab Invest. 2021 Aug;101(8):1071-1083. doi: 10.1038/s41374-021-00598-2. Epub 2021 Apr 19. Lab Invest. 2021. PMID: 33875793
-
Strategy for Treatment of Infected Diabetic Foot Ulcers.Acc Chem Res. 2021 Mar 2;54(5):1080-1093. doi: 10.1021/acs.accounts.0c00864. Epub 2021 Feb 17. Acc Chem Res. 2021. PMID: 33596041 Review.
-
Regulation of NcRNA-protein binding in diabetic foot.Biomed Pharmacother. 2023 Apr;160:114361. doi: 10.1016/j.biopha.2023.114361. Epub 2023 Feb 6. Biomed Pharmacother. 2023. PMID: 36753956 Review.
Cited by
-
Landscape of the epigenetic regulation in wound healing.Front Physiol. 2022 Aug 11;13:949498. doi: 10.3389/fphys.2022.949498. eCollection 2022. Front Physiol. 2022. PMID: 36035490 Free PMC article. Review.
-
The Emerging Role of Major Regulatory RNAs in Cancer Control.Front Oncol. 2019 Sep 24;9:920. doi: 10.3389/fonc.2019.00920. eCollection 2019. Front Oncol. 2019. PMID: 31608229 Free PMC article. Review.
-
The Emerging Therapeutic Targets for Scar Management: Genetic and Epigenetic Landscapes.Skin Pharmacol Physiol. 2022;35(5):247-265. doi: 10.1159/000524990. Epub 2022 Jun 13. Skin Pharmacol Physiol. 2022. PMID: 35696989 Free PMC article. Review.
-
An Optimized and Advanced Algorithm for the Quantification of Immunohistochemical Biomarkers in Keratinocytes.JID Innov. 2024 Feb 16;4(3):100270. doi: 10.1016/j.xjidi.2024.100270. eCollection 2024 May. JID Innov. 2024. PMID: 38756235 Free PMC article.
-
One Molecule, Many Faces: Repositioning Cardiovascular Agents for Advanced Wound Healing.Molecules. 2024 Jun 20;29(12):2938. doi: 10.3390/molecules29122938. Molecules. 2024. PMID: 38931002 Free PMC article. Review.
References
-
- Gould L., Abadir P., Brem H., Carter M., Conner-Kerr T., Davidson J., DiPietro L., Falanga V., Fife C., Gardner S., Grice E., Harmon J., Hazzard W. R., High K. P., Houghton P., et al. (2015) Chronic wound repair and healing in older adults: current status and future research. J. Am. Geriatrics Society 63, 427–438 10.1111/jgs.13332 - DOI - PMC - PubMed
-
- Asai J., Takenaka H., Hirakawa S., Sakabe J., Hagura A., Kishimoto S., Maruyama K., Kajiya K., Kinoshita S., Tokura Y., and Katoh N. (2012) Topical simvastatin accelerates wound healing in diabetes by enhancing angiogenesis and lymphangiogenesis. Am. J. Pathol. 181, 2217–2224 10.1016/j.ajpath.2012.08.023 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

