Fluconazole-Resistant Candida auris Is Susceptible to Salivary Histatin 5 Killing and to Intrinsic Host Defenses
- PMID: 29158282
- PMCID: PMC5786754
- DOI: 10.1128/AAC.01872-17
Fluconazole-Resistant Candida auris Is Susceptible to Salivary Histatin 5 Killing and to Intrinsic Host Defenses
Abstract
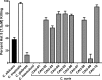
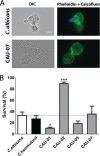
Candida auris is a newly identified species causing invasive candidemia and candidiasis. It has broad multidrug resistance (MDR) not observed for other pathogenic Candida species. Histatin 5 (Hst 5) is a well-studied salivary cationic peptide with significant antifungal activity against Candida albicans and is an attractive candidate for treating MDR fungi, since antimicrobial peptides induce minimal drug resistance. We investigated the susceptibility of C. auris to Hst 5 and neutrophils, two first-line innate defenses in the human host. The majority of C. auris clinical isolates, including fluconazole-resistant strains, were highly sensitive to Hst 5: 55 to 90% of cells were killed by use of 7.5 μM Hst 5. Hst 5 was translocated to the cytosol and vacuole in C. auris cells; such translocation is required for the killing of C. albicans by Hst 5. The inverse relationship between fluconazole resistance and Hst 5 killing suggests different cellular targets for Hst 5 than for fluconazole. C. auris showed higher tolerance to oxidative stress than C. albicans, and higher survival within neutrophils, which correlated with resistance to oxidative stress in vitro Thus, resistance to reactive oxygen species (ROS) is likely one, though not the only, important factor in the killing of C. auris by neutrophils. Hst 5 has broad and potent candidacidal activity, enabling it to combat MDR C. auris strains effectively.
Keywords: Candida auris; histatin; innate host defense; neutrophils.
Copyright © 2018 American Society for Microbiology.
Figures



References
-
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK. 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi:10.1056/NEJMoa1306801. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

