Why the need and how to approach the functional diversity of extracellular vesicles
- PMID: 29158309
- PMCID: PMC5717434
- DOI: 10.1098/rstb.2016.0479
Why the need and how to approach the functional diversity of extracellular vesicles
Abstract
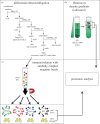
In the past decade, cell-to-cell communication mediated by exosomes has attracted growing attention from biomedical scientists and physicians, leading to several recent publications in top-tier journals. Exosomes are generally defined as secreted membrane vesicles, or extracellular vesicles (EVs), corresponding to the intraluminal vesicles of late endosomal compartments, which are secreted upon fusion of multi-vesicular endosomes with the cell's plasma membrane. Cells, however, were shown to release other types of EVs, for instance, by direct budding off their plasma membrane. Some of these EVs share with exosomes major biophysical and biochemical characteristics, such as size, density and membrane orientation, which impose difficulties in their efficient separation. Despite frequent claims in the literature, whether exosomes really display more important patho/physiological functions, or are endowed with higher potential as diagnostic or therapeutic tools than other EVs, is not yet convincingly demonstrated. In this opinion article, we describe reasons for this lack of precision knowledge in the current stage of the EV field, we review recently described approaches to overcome these caveats, and we propose ways to improve our knowledge on the respective functions of distinct EVs, which will be crucial for future development of well-designed EV-based clinical applications.This article is part of the discussion meeting issue 'Extracellular vesicles and the tumour microenvironment'.
Keywords: cancer; exosomes; extracellular vesicles; multi-vesicular endosomes.
© 2017 The Authors.
Conflict of interest statement
We declare we have no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

